Share this post:
Author: Brent N. Reed, PharmD, BCPS-AQ Cardiology, FAHA
In a June 2017 editorial in JACC Heart Failure, cardiologist and researcher Christopher O’Connor reflected on several recent trials enrolling patients with acute decompensated heart failure (ADHF), in which pharmacologic therapy failed to confer significant improvements in clinical outcomes.1 The three agents to join the list of failed ADHF therapies included the natriuretic peptide ularitide, the relaxin-2 analogue serelaxin, and high-dose spironolactone (100 mg/day).2–4 However, upon reviewing the results of these trials in greater depth, I am left wondering if they represent failures of the drugs themselves or limitations in study methodology.
Many of the recently published ADHF trials, in contrast with studies conducted in patients with chronic heart failure, do not distinguish between heart failure with reduced ejection fraction (HFrEF) and heart failure with preserved ejection fraction (HFpEF) (Table 1). Most of the pharmacologic therapies with proven benefit in chronic HFrEF, including angiotensin-converting enzyme (ACE) inhibitors, angiotensin II receptor blockers (ARBs) and beta blockers, have failed to show similar benefits in those with HFpEF. Why should we expect the treatment of ADHF to be any different? Indeed, there are several reasons to believe that these populations not only present differently in ADHF but that they may also respond differently to cornerstones of therapy in ADHF, including diuretics and vasodilators.
Table 1. Patient Ejection Fraction in Recent Randomized Controlled Trials of Acute Decompensated Heart Failure
| Trial | Year | Agent Studied | Patients | Mean EF (%) | Patients with Preserved EF* |
| ASCEND-HF5 | 2011 | Nesiritide | 7147 | NR | 19.9% |
| DOSE6 | 2011 | Loop diuretics | 308 | 34.8% | 27.0% |
| RELAX-AHF7 | 2012 | Serelaxin | 1161 | 38.7% | 45.0% |
| ROSE-AHF8 | 2013 | Dopamine or nesiritide | 360 | 31.6% | 24.4% |
| TACTICS-HF9 | 2017 | Tolvaptan | 257 | 33.0% | 25.0% |
| TRUE-AHF2 | 2017 | Ularitide | 2157 | NR | 34.8% |
*Not all trials used the same ejection fraction threshold; some used 40% whereas others used 50%
Abbreviations: EF ejection fraction, NR not reported
First, most ADHF trials enroll patients based on the presence of signs and/or symptoms of congestion. However, there are data to suggest that the distribution of excess volume is different in patients with HFrEF versus HFpEF, and that there is discordance between clinical assessment of volume overload and actual congestion in patients with HFpEF but not HFrEF.
In an elegant pilot study published last year, volume profiles among 55 patients with ADHF (35 patients with HFrEF and 20 patients with HFpEF) were studied using a radiolabeled albumin tracer.10 Despite significant inter-patient variability, different volume profiles appeared to emerge between patients with HFrEF and HFpEF. Patients with HFpEF were slightly more likely to be euvolemic at presentation and had less intravascular but more interstitial volume expansion compared to those with HFrEF. Although both subgroups remained intravascularly volume overloaded at discharge despite comparable doses of diuretic therapy, patients with HFpEF lost more interstitial volume relative to intravascular volume (indicating greater fluid mobilization) than those with HFrEF.
Differences in these volume profiles may help explain the discordance between diuretic management in patients with HFpEF who received pulmonary artery (PA) pressure monitoring in the CHAMPION trial versus those whose volume status was assessed by clinical status alone.11 Although the benefits of PA pressure monitoring were similar in HFrEF and HFpEF patients, the management of diuretic therapies differed significantly between monitored and non-monitored patients in HFrEF and HFpEF subgroups. Among patients with HFpEF, those receiving PA pressure monitoring received a mean 50-mg increase in diuretic dose from baseline compared to non-monitored patients (p = 0.0045). In contrast, changes in diuretic dose among HFrEF patients were no different between patients receiving PA monitoring and those who did not (18.9 versus 18.0, p = 0.91). These differences suggest the value that PA pressure monitoring offered in patients with HFpEF and may help explain the reduction in heart failure hospitalizations among this subgroup. Additionally, they demonstrate the challenge of assessing congestion in patients with HFpEF based on clinical assessment alone, and why the presence of signs or symptoms of volume congestion may be a poor criterion for inclusion into ADHF trials.
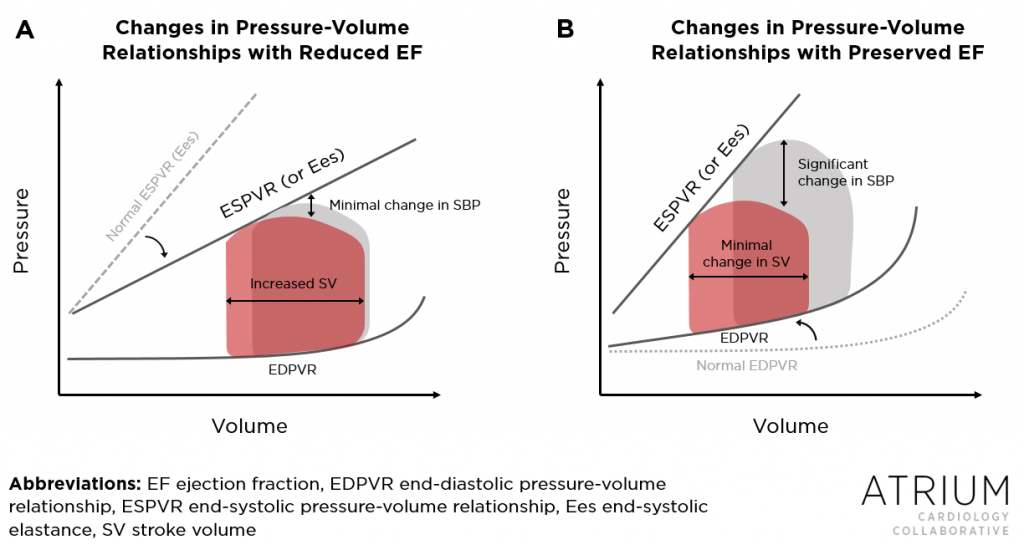
Second and perhaps more importantly, there are pathophysiologic reasons for why patients with HFpEF may respond differently to the agents commonly being studied in ADHF trials. In HFrEF, left ventricular remodeling most commonly involves dilatation, resulting in enlarged chamber size. However, in HFpEF, hypertrophy is more common, resulting in increased wall thickness but relatively unchanged chamber size. These differences in morphology confer differences in how stroke volume (and thus cardiac output) responds to alterations in both preload and afterload (Figure 1).12 In HFpEF, the decreased compliance that results from stiffening of the left ventricle increases the slope of the end-diastolic pressure-volume relationship (seen in Figure 1B). Consequently, stroke volume in patients with HFpEF is more sensitive to the alterations in preload that may result from diuresis or venodilation. In HFrEF, however, the end-diastolic relationship is relatively unchanged and patients can tolerate significant reductions in preload without compromising stroke volume (Figure 1A).
Differences in how patients with HFpEF or HFrEF respond to alterations in afterload are a result of differences in ventricular-arterial relationships. This is commonly depicted as the end-systolic elastance (Ees), as shown in Figure 1. In HFrEF, reductions in afterload generally lead to improvements in left ventricular performance (with the exception of those with end-stage disease in whom contractile reserve is limited). This relationship (i.e., a relatively flatter Ees, as shown in Figure 1A) explains the phenomenon by which administration of a vasodilator to patients with ADHF in the setting of HFrEF may have minimal impact on blood pressure, as reductions in systemic vascular resistance are met with improvements in cardiac output and thus mean arterial pressure. However, in HFpEF, the ventricular-arterial relationship is relatively unchanged (i.e., a relatively steep Ees), meaning that reductions in afterload confer minimal if any improvements in stroke volume but may significantly reduce blood pressure (Figure 1B). (If you want to learn more about pressure-volume relationships, the video series at Khan Academy is excellent).
Figure 1. In HFrEF (A), the slope of the end-systolic pressure volume-relationship (ESPVR) or end-systolic elastance (Ees) is flatter as a result of reduced contractility. Reductions in afterload therefore result in minimal changes in systolic blood pressure (SBP) but lead to improvements in stroke volume. In HFpEF (B), the slope of ESPVR is relatively unchanged from normal patients, leading to substantial decreases in SBP with any reductions in afterload. The effect of alterations in preload can also be described by the Figure. In HFrEF (A), the end-diastolic pressure-volume relationship (EDPVR) is relatively unchanged. Reductions in preload therefore confer minimal changes in stroke volume. In HFpEF (B) however, the EDPVR is steeper as a result of reduced compliance, resulting in a ventricle that is more sensitive to changes in preload. (Figure adapted from the findings in Reference 12).
Therefore, as can be inferred from Figure 1, differences in pressure-volume relationships in patients with HFrEF and HFpEF pre-dispose them to respond differently to drugs that alter preload (diuretics and venodilators) and afterload (arterial vasodilators). Indeed, data from small trials and sub-analyses of larger randomized controlled trials appear to support these concepts.
In one of the most commonly cited studies to investigate this phenomenon, researchers measured hemodynamic changes following administration of intravenous sodium nitroprusside in patients with HFrEF (n=174) and HFpEF (n=83) undergoing right heart catheterization.12 Following nitroprusside administration, reductions in pulmonary capillary wedge pressure (PCWP) and LV end-diastolic pressures (LVEDP) (i.e., measures of preload) were similar between patients with HFrEF and HFpEF (-9±6 and -9±6 mmHg respectively for PCWP, p = 0.70, and -8±5 and -9±6 mmHg respectively for LVEDP p = 0.90). However, improvements in cardiac output and stroke volume were over two-fold greater among patients with HFrEF (p < 0.0001). In fact, patients with HFpEF were nearly four times more likely to experience a decrease in stroke volume (p < 0.0001), as well as an over two-fold greater reduction in systolic blood pressure (-51 versus -22 mmHg in those with HFrEF, p < 0.0001).
Signals to support a difference in clinically meaningful outcomes can be found in subgroup analyses of several large randomized controlled trials. For example, in the ASCEND-HF trial comparing nesiritide and placebo in ADHF, subgroup analysis of the co-primary endpoint (dyspnea relief at 6 and 24 hours) indicates that patients with HFrEF may have benefitted from nesiritide whereas those with HFpEF did not.5 In the RELAX-AHF trial comparing serelaxin to placebo in ADHF, patients experiencing dyspnea relief at 24 hours was no different among those who received active drug.7 However, in a sub-analysis analyzing patients with HFrEF and HFpEF separately, serelaxin appeared to be more effective at relieving dyspnea in patients with HFpEF at 6, 12, and 24 hours (p-value for interaction = 0.030).13 A differential response to low-dopamine or low-dose nesiritide in patients with HFrEF or HFpEF was also evident in the ROSE-AHF trial.8 In a sub-analysis comparing these two subgroups, both drugs were associated with increases in urine volume among HFrEF but not HFpEF patients.14 In patients randomized to low-dose dopamine, active drug improved clinical outcomes among patients with HFrEF and worsened them among those with HFpEF. No differences in clinical outcomes were observed among those randomized to low-dose nesiritide.
I was unable to find a subgroup analysis of patients with HFrEF and HFpEF from the DOSE trial. This information could be important for individualizing drug therapy in these two subgroups, as differences in pressure-volume relationships should also be expected to alter how patients with HFrEF versus HFpEF respond to intravenous loop diuretic therapy. In a previous blog entry, I discussed how this may be particularly true when deciding between bolus and continuous infusion administration.
In summary, although patients with ADHF most commonly present with signs and symptoms of congestion, the underlying pathophysiology may be different depending on whether patients have HFrEF or HFpEF. Even among patients with HFpEF, one can reasonably argue that the condition is itself heterogeneous and a one-size-fits-all strategy may not work in any sample of HFpEF patients with ADHF. At minimum, we should revisit the criteria for inclusion into ADHF trials and either restrict analyses to one of the two subgroups, or at least power the trials to analyze these subgroups individually. Although the enrollment windows for trials of ADHF are often short (usually 48 hours or less), an ejection fraction can be easily obtained with bedside echocardiography. Without making this important distinction, it is unlikely that we will ever identify a pharmacologic therapy that works equally well across such a heterogeneous population. Instead, we may be obscuring the benefit of therapy in one of these subgroups. Worse yet, we may also be masking the harm.
 |
Brent N. Reed, PharmD, BCPS-AQ Cardiology, FAHADr. Reed is an assistant professor in the Department of Pharmacy Practice and Science at the University of Maryland School of Pharmacy, and practices as a clinical pharmacy specialist in advanced heart failure at the University of Maryland Medical Center in Baltimore, MD. Follow him on his website or on Twitter @brentnreed. |
References
- O’Connor CM. Development of Acute Decompensated Heart Failure Therapies: Is the Journey Over? JACC Heart Fail. 2017 Jun;5(6):464–5.
- Packer M, O’Connor C, McMurray JJV, Wittes J, Abraham WT, Anker SD, et al. Effect of Ularitide on Cardiovascular Mortality in Acute Heart Failure. N Engl J Med. 2017 18;376(20):1956–64.
- Serelaxin Again Bombs in Acute Heart Failure: RELAX-AHF-2 [Internet]. Medscape. [cited 2017 Jun 6]. Available from: http://www.medscape.com/viewarticle/877654
- No Added Value to Spironolactone in Acute HF: ATHENA-HF [Internet]. Medscape. [cited 2017 Jun 6]. Available from: http://www.medscape.com/viewarticle/872517
- O’Connor CM, Starling RC, Hernandez AF, Armstrong PW, Dickstein K, Hasselblad V, et al. Effect of nesiritide in patients with acute decompensated heart failure. N Engl J Med. 2011 Jul 7;365(1):32–43.
- Felker GM, Lee KL, Bull DA, Redfield MM, Stevenson LW, Goldsmith SR, et al. Diuretic strategies in patients with acute decompensated heart failure. N Engl J Med. 2011 Mar 3;364(9):797–805.
- Teerlink JR, Cotter G, Davison BA, Felker GM, Filippatos G, Greenberg BH, et al. Serelaxin, recombinant human relaxin-2, for treatment of acute heart failure (RELAX-AHF): a randomised, placebo-controlled trial. Lancet Lond Engl. 2013 Jan 5;381(9860):29–39.
- Chen HH, Anstrom KJ, Givertz MM, Stevenson LW, Semigran MJ, Goldsmith SR, et al. Low-Dose Dopamine or Low-Dose Nesiritide in Acute Heart Failure With Renal Dysfunction: The ROSE Acute Heart Failure Randomized Trial. JAMA J Am Med Assoc. 2013 Nov 18;
- Felker GM, Mentz RJ, Cole RT, Adams KF, Egnaczyk GF, Fiuzat M, et al. Efficacy and Safety of Tolvaptan in Patients Hospitalized With Acute Heart Failure. J Am Coll Cardiol. 2017 Mar 21;69(11):1399–406.
- Miller WL, Mullan BP. Volume Overload Profiles in Patients With Preserved and Reduced Ejection Fraction Chronic Heart Failure: Are There Differences? A Pilot Study. JACC Heart Fail. 2016 Jun;4(6):453–9.
- Adamson PB, Abraham WT, Bourge RC, Costanzo MR, Hasan A, Yadav C, et al. Wireless pulmonary artery pressure monitoring guides management to reduce decompensation in heart failure with preserved ejection fraction. Circ Heart Fail. 2014 Nov;7(6):935–44.
- Schwartzenberg S, Redfield MM, From AM, Sorajja P, Nishimura RA, Borlaug BA. Effects of vasodilation in heart failure with preserved or reduced ejection fraction implications of distinct pathophysiologies on response to therapy. J Am Coll Cardiol. 2012 Jan 31;59(5):442–51.
- Filippatos G, Teerlink JR, Farmakis D, Cotter G, Davison BA, Felker GM, et al. Serelaxin in acute heart failure patients with preserved left ventricular ejection fraction: results from the RELAX-AHF trial. Eur Heart J. 2014 Apr;35(16):1041–50.
- Wan S-H, Stevens SR, Borlaug BA, Anstrom KJ, Deswal A, Felker GM, et al. Differential Response to Low-Dose Dopamine or Low-Dose Nesiritide in Acute Heart Failure With Reduced or Preserved Ejection Fraction: Results From the ROSE AHF Trial (Renal Optimization Strategies Evaluation in Acute Heart Failure). Circ Heart Fail. 2016 Aug;9(8).
Share this post: