Share this post:
Author: Brent N. Reed, PharmD, BCCP, FAHA
One of the questions that remained unanswered after the PARADIGM-HF trial was whether the angiotensin receptor / neprilysin inhibitor (ARNI) sacubitril/valsartan could be safely initiated in patients with acute decompensated heart failure (ADHF) who had not first been stabilized on an angiotensin-converting enzyme (ACE) inhibitor or angiotensin II receptor blocker (ARB). Much of the reluctance to use ARNI in this setting was due to the increased risk of symptomatic hypotension observed with sacubitril/valsartan compared to enalapril (14.0% vs. 9.2% of patients, respectively, p < 0.001).1
These concerns were mostly laid to rest following the results of the TRANSITION trial, which were presented at the 2018 Heart Failure Society of America meeting in September (full publication not yet available), and PIONEER-HF, which was presented at the 2018 American Heart Association Scientific Sessions and simultaneously published in the New England Journal of Medicine.2,3
In TRANSITION, 1002 patients with ADHF were randomized to sacubitril/valsartan prior to discharge or within 14 days post-discharge, and clinicians were instructed to up-titrate therapy toward the target dose of 97/103 mg twice daily.2 Comparable numbers of patients were able to achieve target dose in the two groups (62% and 68% in the pre- vs. post-discharge groups, respectively) and adverse events were also similar. In particular, hypotension occurred in 12.3% and 9.1% of patients in the pre- and post-discharge groups, respectively (p = ns).
In PIONEER-HF, 881 patients with ADHF and hemodynamic stabilization (systolic blood pressure > 100 mmHg for > 6 hours, off vasodilators or inotropes, and not requiring increases in diuretic therapy) were randomized to sacubitril/valsartan or enalapril. Sacubitril/valsartan improved the primary endpoint of change in N-terminal pro-B-type natriuretic peptide through weeks 4 and 8, but arguably the most important endpoint was the similar rates of adverse events in the two groups. In particular, symptomatic hypotension occurred in 15.0% and 12.7% of patients in the sacubitril/valsartan and enalapril groups, respectively (p = ns). Rates of worsening renal function, hyperkalemia, and angioedema were also similar between groups.
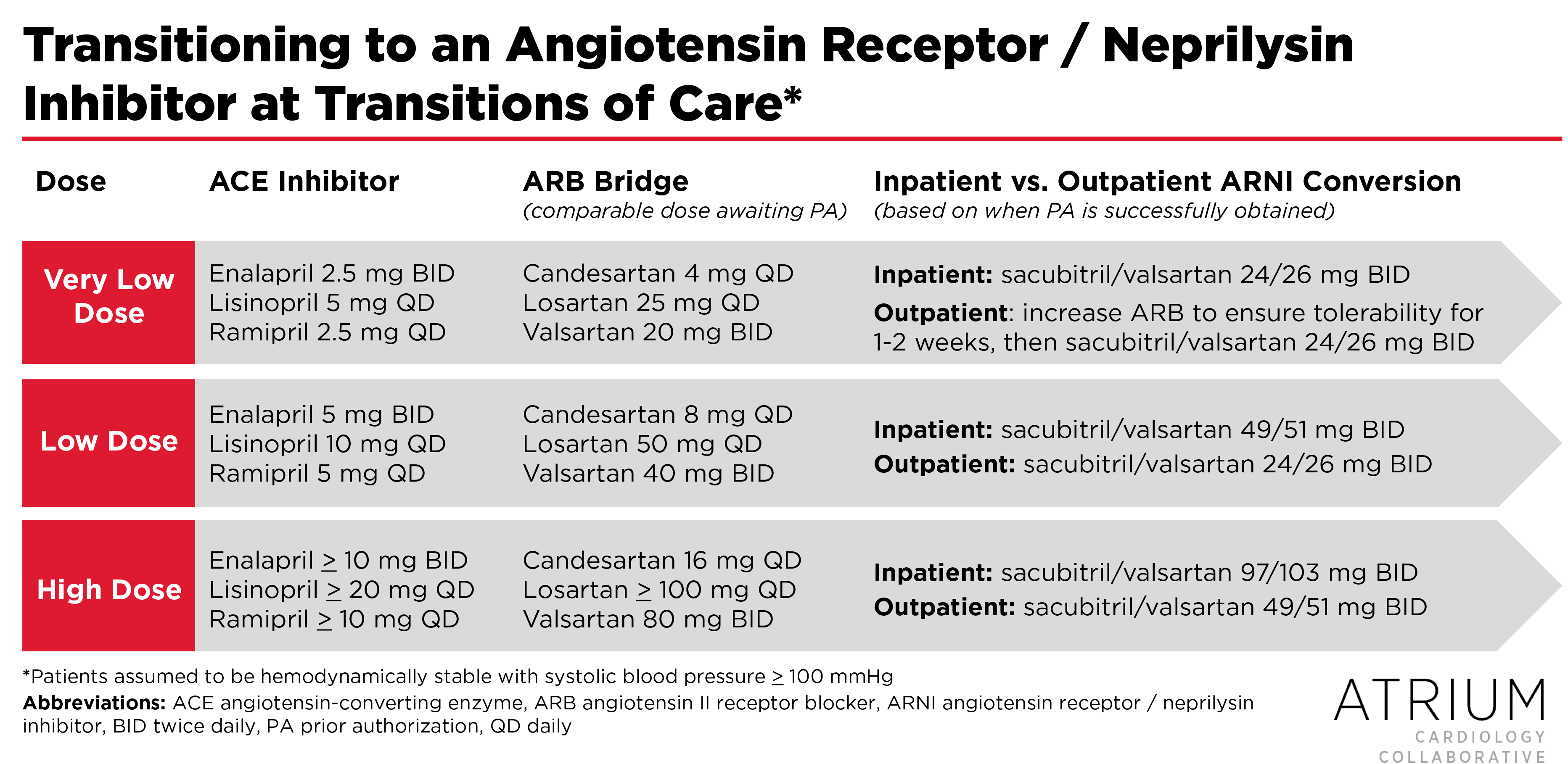
Now that the concerns regarding ARNI intolerability in patients with stable ADHF have largely been assuaged, the key question remaining is how best to transition patients to sacubitril/valsartan, as this was executed differently in PARADIGM-HF and PIONEER-HF. In PARADIGM-HF, patients with chronic heart failure who were able to tolerate target dose enalapril were transitioned to a lower dose of sacubitril/valsartan (49/51 mg) for a two-week run-in period (after a 36-hour ACE inhibitor washout) prior to being increased to 97/103 mg twice daily for the remainder of the study. The labeling for sacubitril/valsartan is consistent with this practice, and also suggests that patients on lower doses of an ACE inhibitor (enalapril < 5 mg twice daily or an equivalent dose of an alternative ACE inhibitor) should be initiated on an even lower dose of sacubitril/valsartan (24/26 mg twice daily) prior to up-titration.
However, patients in PIONEER-HF were randomized to what are generally considered equivalent doses of sacubitril/valsartan and enalapril (i.e., without a half-dose run-in period), based on their baseline systolic blood pressure at the time of randomization. Patients with systolic blood pressure of 100-119 mmHg were randomized to sacubitril/valsartan 24/26 mg twice daily or enalapril 2.5 mg twice daily, whereas those with systolic blood pressure > 120 mmHg were randomized to sacubitril/valsartan 49/51 mg or enalapril 5 mg twice daily. Although patients with ADHF are more sensitive to hemodynamic perturbations, the more aggressive initiation of therapy in PIONEER-HF seems reasonable given the ability to closely monitor patient response.
The other complicating factor for initiating sacubitril/valsartan in patients with ADHF is that many insurers still require prior authorization (PA), a process that may require days to complete, particularly since many inpatient care teams are often poorly equipped to handle it (for example, an outpatient community pharmacy is required to submit claims and may not be well-integrated within the facility). Keeping patients hospitalized solely for the purpose of obtaining a PA is a suboptimal use of health care resources, but sending patients home without any renin-angiotensin-aldosterone system inhibition (due to the need for a 36-hour ACE inhibitor washout) also places them at risk for worsening heart failure and hospital readmission. Communicating the ARNI transition plan to the clinician assuming care for the patient adds even further complexity to the process.
One way to facilitate the transition to ARNI therapy in patients who are on an ACE inhibitor is to bridge with an ARB while awaiting PA approval. To provide guidance on this process, we’ve developed the following algorithm, which uses an ARB bridge and integrates the transition practices used in PARADIGM-HF and PIONEER-HF.
In summary, bridging patients with an ARB over the 36-hour washout period (while awaiting PA approval) prevents the need to coordinate this challenging process across the transition of care. If the PA is successfully obtained prior to discharge, patients can be transitioned from an ARB to the higher doses of sacubitril/valsartan used in PIONEER-HF. Alternatively, if the PA cannot be obtained prior to discharge, the patient can be discharged on an ARB and then transitioned to sacubitril/valsartan as an outpatient using the less aggressive dosing strategy of PARADIGM-HF (since tolerability cannot be monitored as closely in the outpatient setting). In both cases, patients should be titrated every two weeks as tolerated to the recommended target dose of 97/103 mg twice daily.
 |
Brent N. Reed, PharmD, BCCP, FAHADr. Reed is an associate professor in the Department of Pharmacy Practice and Science at the University of Maryland School of Pharmacy, and practices as a clinical pharmacy specialist in advanced heart failure at the University of Maryland Medical Center in Baltimore, MD. Follow him on his website or on Twitter @brentnreed. |
References
- McMurray JJV, Packer M, Desai AS, Gong J, Lefkowitz MP, Rizkala AR, et al. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med. 2014 Sep 11;371(11):993–1004.
- Stiles S. Safe to Start Sacubitril/Valsartan Predischarge in Acute HF [Internet]. Medscape Cardiology. 2018 [cited 2018 Nov 30]. Available from: https://www.medscape.com/viewarticle/902128
- Velazquez EJ, Morrow DA, DeVore AD, Duffy CI, Ambrosy AP, McCague K, et al. Angiotensin–Neprilysin Inhibition in Acute Decompensated Heart Failure. N Engl J Med [Internet]. 2018 Nov 11 [cited 2018 Nov 30]; Available from: https://doi.org/10.1056/NEJMoa181285
Share this post: