Share this post:
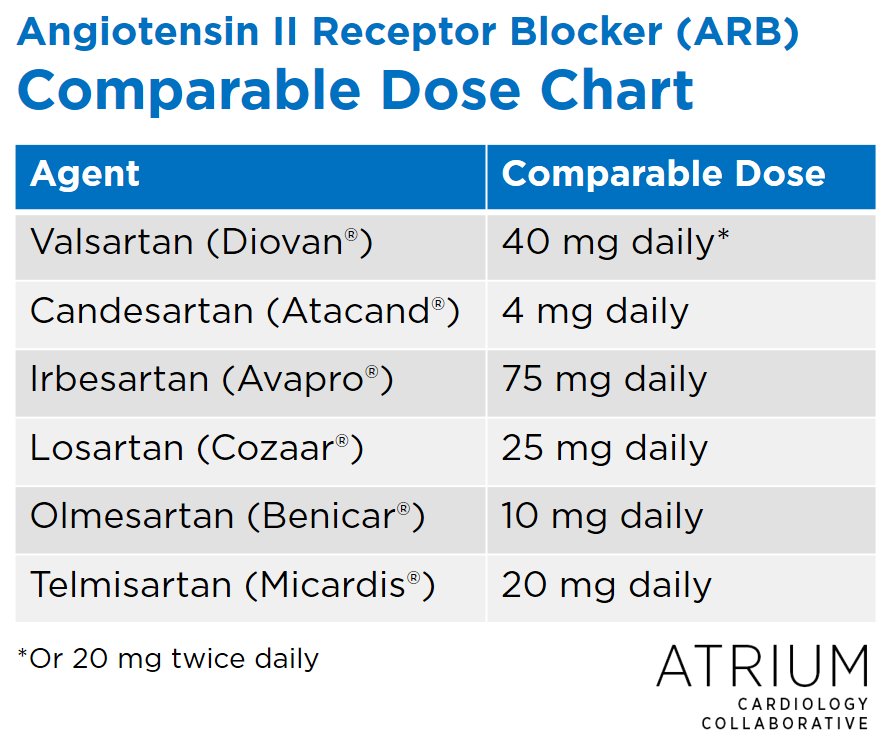
The US Food and Drug Administration (FDA) has recalled several angiotensin II receptor blocker-containing products due to the presence of chemical impurities. This list includes valsartan, losartan and irbesartan products. Notably, the recall does not involve sacubitril/valsartan (Entresto). Patients taking the affected products are encouraged to contact their prescriber or pharmacist to switch to an alternative agent, but should not stop taking therapy abruptly. To help clinicians with selecting an alternative ARB, we wanted to share the following table of comparable doses:
| Agent | Comparable Dose |
| Valsartan (Diovan®) | 40 mg daily* |
| Candesartan (Atacand®) | 4 mg daily |
| Irbesartan (Avapro®) | 75 mg daily |
| Losartan (Cozaar®) | 25 mg daily |
| Olmesartan (Benicar®) | 10 mg daily |
| Telmisartan (Micardis®) | 20 mg daily |
*Or 20 mg twice daily
You can also help us spread the word by sharing our image below:
Note: This post is an updated version of an entry originally written in September 2018.
Share this post: