Share this post:
Author: Zachary R. Noel, PharmD, BCCP
The use of direct oral anticoagulants (DOACs) in patients with atrial fibrillation (AF) and end stage renal disease (ESRD) remains controversial. It’s no secret that patients with ESRD were not studied in landmark clinical trials such as ROCKET-AF, ARISTOTLE, and RE-LY.1 Thus, at this time, clinicians are forced to examine retrospective studies and/or pharmacokinetic data to make a decision – something that we in cardiology don’t often utilize because of overshadowing data from large, randomized clinical trials. Of the DOACs on the market today, rivaroxaban and apixaban have been studied in ESRD because of their relatively low renal clearance (i.e., 25-35%).2,3 Apixaban is the most widely used in ESRD due to its more favorable pharmacokinetic profile, and is actively being studied in prospective clinical trials.4-6 For these reasons, apixaban is the focus of this blog post.
The Case for a Better Alternative
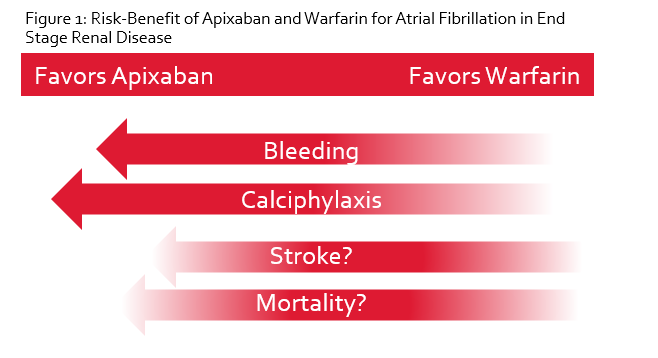
As outlined in Part I of this series, the decision to anticoagulate at all in patients with ESRD and AF is contentious. This is largely due to the questionable benefit observed over the years with warfarin in ESRD. In fact, several studies have shown no difference in ischemic stroke rates with warfarin but an increase in intracranial hemorrhage, yielding net harm.7-9 Additionally, calciphylaxis is a dreaded complication in patients with ESRD, and warfarin use is associated with a 10-fold increase in developing this problem.10 Taken altogether, it’s not difficult to see why no anticoagulation may be better than warfarin. However, it’s possible that an alternative anticoagulant such as apixaban may fare better than either no anticoagulation or warfarin. In the absence of robust clinical trials, we must rely on the available evidence and think outside the box to balance the risk of stroke and bleeding in this complex population.
Did You Know?
Apixaban should NOT be empirically dose reduced for patient with ESRD. Dose reduction for AF still requires one additional criterion (i.e., weight ≤ 60 kg or age ≥ 80 years old).
Pharmacokinetic Data of Apixaban in ESRD
In 2014 the FDA updated package labeling for apixaban to include information on dosing in patients with ESRD.3 This update was based on a single dose pharmacokinetic study in eight patients, which showed a 36% increase in the area under the curve (AUC) for ESRD patients receiving apixaban 5 mg compared to those with normal renal function.11 More recently, Mavrakanas et al published a steady state pharmacokinetic study in seven patients on dialysis receiving apixaban 2.5 mg twice daily.12 Five of these patients underwent a washout period and then crossed over to receive apixaban 5 mg twice daily. There was no active comparator, and the reference group was based on prior pharmacokinetic data in patients without advanced kidney disease. The results of the study revealed that patients on hemodialysis receiving apixaban 2.5 mg twice daily had a mean AUC ~33% less than the comparator group of patients without kidney disease receiving 5 mg twice daily. In contrast, the five ESRD patients who received apixaban 5 mg twice daily had a mean AUC ~45% higher than that of healthy comparators receiving an equivalent dose. While the authors conclude that apixaban 2.5 mg twice daily should be used in patients on dialysis, it’s important to recognize that all seven patients in the study who received 2.5 mg twice daily had an AUC that was at or below the 10th percentile for healthy comparators receiving 5 mg twice daily. This suggests that empirically dose reducing in spite of the package labeling recommendations could result in subtherapeutic concentrations of apixaban and increase the risk of stroke. In contrast, those who received 5 mg twice daily were more likely to fall within the 10-90th percentile of healthy volunteers; however, two patients did have AUC concentrations well above the 90th percentile, which highlights the substantial interpatient variability as well as the risk for accumulation at steady state with 5 mg twice daily.
Clinical Evidence Comparing Apixaban to Warfarin
There have been a few retrospective studies comparing apixaban and warfarin in patients with ESRD and AF. Two small, single center analyses showed that apixaban and warfarin had similar rates of major bleeding in ESRD.13, 14 Each were underpowered to detect differences in stroke and included patients with alternative indications (i.e., venous thromboembolism), making it difficult to draw strong conclusions. Recently, a large retrospective, matched cohort study of 25,000 patients with ESRD and AF were analyzed from a Medicare registry.4 Patients were included only if they were newly prescribed an anticoagulant within the last year and were receiving dialysis. Patients were matched to apixaban and warfarin in a 1:3 fashion according to baseline characteristics. Approximately half of the patients in the apixaban group received 5 mg and the other half received 2.5 mg twice daily. Overall, observed rates of stroke and systemic embolism were similar between the two groups; however, major bleeding was significantly less in the apixaban arm (hazard ratio [HR] 0.72; 95% confidence interval [CI] 0.59-0.87; p < 0.001). Interestingly, those who received the 5 mg dose had lower rates of stroke and systemic embolism, major bleeding, and mortality. Those who received 2.5 mg had lower rates of bleeding but a similar stroke rate and mortality. As encouraging as these results may be, there are several important limitations. First, there was not a comparator group of patients that received no anticoagulation. Second, more than two-thirds of patients discontinued the anticoagulant during the first year. Given the nature of the study, it was not possible to identify the reason for therapy discontinuation. Lastly, information on weight was not available; thus, it was not possible to evaluate whether patients receiving 2.5 mg twice daily were appropriately dose reduced.
Taken altogether, observational data consistently demonstrates similar or decreased risk of stroke and less bleeding with apixaban compared to warfarin. A recent meta-analysis corroborated these findings.15 Two prospective trials are ongoing and results are expected in 2019 or 2020.5, 6 Until then, it will remain unclear whether apixaban is definitively superior to warfarin; however, we can safely and reasonably assume based on current evidence that apixaban is no worse than warfarin.
Conclusions & Recommendations
In conclusion, the evidence for using apixaban in ESRD and AF is insufficient to make strong recommendations. Nonetheless, it is important to collectively consider available data and place it in context with warfarin, which not only has yet to consistently demonstrate a reduction in ischemic stroke in ESRD, but also increases the risk of major bleeding and calciphylaxis while consuming additional time and money for both patients and the healthcare system. Based on available data, apixaban is a compelling and reasonable anticoagulant to use in ESRD and has been shown in retrospective studies to be no less efficacious than warfarin but with less adverse drug effects, including major bleeding.
 |
Zachary R. Noel, PharmD, BCCP
|
References
- Nishimura M, Hsu JC. Non-Vitamin K Antagonist Oral Anticoagulants in Patients With Atrial Fibrillation and End-Stage Renal Disease. The American Journal of Cardiology. 2018;121(1):131-140. doi:10.1016/j.amjcard.2017.09.030.
- Package insert. Rivaroxaban (Xarelto®). Accessed January 16, 2019
- Package insert. Apixaban (Eliquis®). Accessed January 16, 2019
- Siontis KC, Zhang X, Eckard A, et al. Outcomes Associated With Apixaban Use in Patients With End-Stage Kidney Disease and Atrial Fibrillation in the United States. Circulation. 2018;138(15):1519-1529. doi:10.1161/CIRCULATIONAHA.118.035418.
- clinicaltrials.gov. Compare Apixaban and Vitamin-K Antagonists in Patients With Atrial Fibrillation (AF) and End-Stage Kidney Disease (ESKD) (AXADIA). Accessed January 16, 2019.
- clinicaltrials.gov. Trial to Evaluate Anticoagulation Therapy in Hemodialysis Patients With Atrial Fibrillation (RENAL-AF). Accessed January 16, 2019.
- Nochaiwong S, Ruengorn C, Awiphan R, Dandecha P, Noppakun K, Phrommintikul A. Efficacy and safety of warfarin in dialysis patients with atrial fibrillation: a systematic review and meta-analysis. Open Heart (2053-3624). 2016;3(1):1.
- Wang TKM, Sathananthan J, Marshall M, Kerr A, Hood C.Relationships between Anticoagulation, Risk Scores and Adverse Outcomes in Dialysis Patients with Atrial Fibrillation. Heart, Lung and Circulation. 2016;25:243-249. doi:10.1016/j.hlc.2015.08.012.
- Winkelmayer WC, Liu J, Setoguchi S, Choudhry NK. Effectiveness and safety of warfarin initiation in older hemodialysis patients with incident atrial fibrillation. Clinical Journal Of The American Society Of Nephrology: CJASN. 2011;6(11):2662-2668. doi:10.2215/CJN.04550511.
- Hayashi, M., Takamatsu, I., Kanno, Y., Yoshida, T., Abe, T. & Sato, Y.; for the Japanese Calciphylaxis Study Group. (2012) A case‐control study of calciphylaxis in Japanese end‐stage renal disease patients. Nephrology Dialysis Transplantation, 27, 1580–1584.
- Wang X, Tirucherai G, Marbury TC, et al. Pharmacokinetics, pharmacodynamics, and safety of apixaban in subjects with end-stage renal disease on hemodialysis. Journal Of Clinical Pharmacology. 2016;56(5):628-636. doi:10.1002/jcph.628.
- Mavrakanas TA, Samer CF, Nessim SJ, Frisch G, Lipman ML. Apixaban Pharmacokinetics at Steady State in Hemodialysis Patients. Journal of The American Society Of Nephrology: JASN. 2017;28(7):2241-2248. doi:10.1681/ASN.2016090980.
- Stanton BE, Barasch NS, Tellor KB. Comparison of the Safety and Effectiveness of Apixaban versus Warfarin in Patients with Severe Renal Impairment. Pharmacotherapy. 2017;37(4):412-419. doi:10.1002/phar.1905.
- Sarratt SC, Nesbit R, Moye R. Safety Outcomes of Apixaban Compared With Warfarin in Patients With End-Stage Renal Disease. The Annals Of Pharmacotherapy. 2017;51(6):445-450. doi:10.1177/1060028017694654.
- Chokesuwattanaskul R, Thongprayoon C, Tanawuttiwat T, Kaewput W, Pachariyanon P, Cheungpasitporn W. Safety and efficacy of apixaban versus warfarin in patients with end‐stage renal disease: Meta‐analysis. Pacing & Clinical Electrophysiology. 2018;41(6):627. http://survey.hshsl.umaryland.edu/?url=http://search.ebscohost.com/login.aspx?direct=true&db=edb&AN=130266573&site=eds-live. Accessed January 23, 2019.
Share this post: