Share this post:
Author: Michael Plazak, PharmD and Kristin Watson, PharmD, BCPS-AQ Cardiology
Prior to directly comparing the different formulations of hydralazine (HYD) and isosorbide dinitrate (ISDN), let us first revist the historical data for these therapies in patients with heart failure with reduced ejection fraction (HFrEF). The routine use of HYD and ISDN in patients with HFrEF dates back to the Vasodilator Heart Failure Trial (V-HeFT I) study published in 1986. Compared to placebo, HYD/ISDN titrated to a target dose of 40 mg and 75 mg, respectively, four times daily improved left ventricular ejection fraction at both 8 weeks and 1 year among patients with New York Heart Association (NYHA) Class II-III heart failure. However, these improvements did not translate into a reduction in mortality at a mean follow-up of 2.3 years.1 In V-HeFT II, enalapril was associated with a lower risk of death at 2 years compared to HYD/ISDN in patients with primarily symptomatic HFrEF.2 Following the publications of V-HeFT I and II, HYD/ISDN was recommended in patients with HFrEF who were unable to receive an angiotensin-converting enzyme inhibitor (ACEi) due to hypotension or renal insufficiency. A nitrate, alone or with hydralazine, could also be added to a regimen consisting of an ACEi, beta blocker, digitalis, and a diuretic.3
Sub-group analyses of V-HeFT I and II suggested that black patients may derive a mortality benefit from HYD/ISDN. These hypothesis-generating findings were confirmed in the African-American Heart Failure trial (A-HeFT) in which a new fixed-dose combination of HYD/ISDN (BiDil®) was shown to reduce the risk of death and heart failure hospitalization and improve quality of life when added to ACEi and beta blocker therapy in self-described black patients with NYHA Class III-IV heart failure.4 Current recommendations for the use of HYD/ISDN are provided in Table 1.
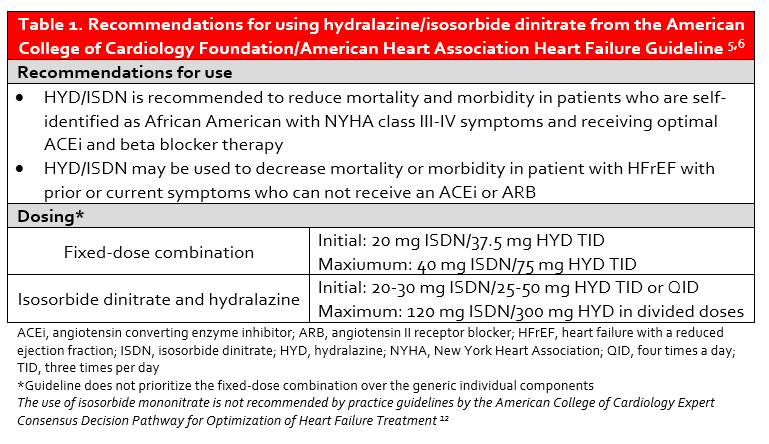
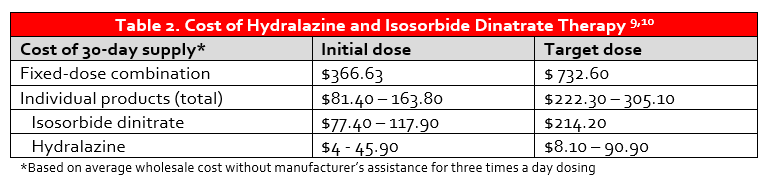
The use of guideline-directed medical therapy (GDMT) in patients with HFrEF remains suboptimal, and this is also true of HYD/ISDN. Despite the clear benefit of fixed-dose combination HYD/ISDN in black patients with NYHA Class III-IV HFrEF, there remains a disconnect in prescribing and overall usage.7,8 The explanation for this dearth of prescribing is largely unknown, but may be related to adherence concerns with thrice daily dosing. To further compound this issue, the cost of BiDil® may be prohibitive as lack of insurance is a predictor of under-usage.7 While the manufacturer discount program can decrease the cost of BiDil® to $10 per month in patients with particular insurance plans and $50 per month in those without insurance coverage, the annual cost of therapy is still high (Table 2).11 Patients are therefore commonly prescribed the individual components of HYD and ISDN, which requires administration of at least six tablets per day.
The results of the landmark A-HeFT trial and current prescribing trends prompt several questions. First, what are the pathophysiologic differences in black patients with HFrEF that result in improved response to vasodilator therapy? Second, does the formulation of nitrate therapy impact the pharmacokinetics, pharmacodynamics, and ultimately clinical outcomes?The use of extended-release ISMN has emerged as a potential alternative for ISDN potentially owing to its once daily dosing and that select doses are on discount prescription plans.10 However, in the 2017 American College of Cardiology Expert Consensus Decision Pathway for Optimization of Heart Failure Treatment, the authors note that ISMN is not recommended by practice guidelines, and only the fixed-dose combination or separate combination of HYD/ISDN should be considered GDMT for heart failure.12 Despite this, a retrospective analysis of black patients with HFrEF and Medicare Part D coverage indicated that 43.9% of outpatient prescriptions for HYD/nitrate therapy were for HYD/ISMN. This was complemented by only 26.8% of patients receiving the fixed-dose combination and 29.3% receiving the individual components of HYD and ISDN.13
In V-HeFT I, the utility of HYD/ISDN was thought to be secondary to a simultaneous improvement in preload by ISDN and afterload by HYD.1 However, the progression of HFrEF has also been associated with dysregulation of the nitroso-redox balance. This ultimately results in excess oxidative stress, thus disrupting the maintenance of nitric oxide.14 ISDN and ISMN are organic nitrates, which fuel the continued signaling of nitric oxide. HYD acts synergistically by inhibiting the production of superoxide, which is also thought to reduce nitrate tolerance. Based on the results from the studies and subgroup analyses mentioned previously, the proposed mechanism for this phenomenon is that the renin-angiotensin system is thought to be less active and nitric oxide bioavailability is lower in this patient population.4,15
There is data assessing the pharmacokinetics of the different HYD/ISDN formulations utilized in V-HeFT I, V-HeFT II, and A-HeFT. Healthy volunteers were randomized to receive identical doses of HYD capsule plus ISDN tablet (V-HeFT I formulation), HYD tablet plus ISDN tablet (V-HeFT II formulation), or fixed-dose HYD/ISDN (A-HeFT formulation). Compared to the V-HeFT I and V-HeFT II formulations of ISDN, the fixed-dose combination exhibited a higher ISDN Cmax, but similar ISDN AUC’s. Additionally, the ISDN and HYD in the fixed-dose combination peaked at nearly the same time, whereas the other two formulations did not demonstrate this phenomenon. The authors ultimately concluded that the three formulations did not demonstrate bioequivalence; however, this was largely driven by differences in HYD pharmacokinetics.16 All of the patients in these pharmacokinetic studies were healthy volunteers and the majority were of Caucasian descent, therefore, it is not practical to extrapolate these results to any meaningful clinical impact in a black patient with HFrEF.
ISMN, the primary active metabolite of ISDN, was not used in any of these landmark studies and its role in HFrEF is not well-established. However, it does effectively increase nitric oxide signaling and conveys several unique pharmacologic benefits compared to ISDN. The extended-release formulation of ISMN does not undergo first-pass metabolism, is nearly 100% bioavailable, and is dosed once daily.17 ISDN on the other hand exhibits extensive first-pass metabolism, a bioavailability of 20-30%, demonstrates high peak concentrations between 30 and 60 minutes, and requires three to four doses per day.18
Only one publication has assessed clinical outcomes with fixed-dose combination HYD/ISDN (BiDil®) compared to HYD/nitrate therapies being prescribed separately (HYD+ISDN and HYD+ISMN). In this propensity-score matched analysis, black Medicare beneficiaries with heart failure and at least 80% 1-year adherence with HYD/nitrate therapy were included. Survival at 1-year was 87.9% with fixed-dose HYD/ISDN compared to 83.0% with HYD+ISDN (p=0.0024) and 88.2% with fixed-dose HYD/ISDN compared to 84.8% with HYD+ISMN (p=0.032).13 While this analysis demonstrated favorable survival at 1-year with fixed-dose HYD/ISDN, there was no means of differentiating patients with HFrEF from those with heart failure with preserved ejection fraction. Other limitations of this study are that prescription refill data does not necessarily provide accurate indications for therapy and 80% adherence with a thrice daily formulation is likely not replicable in clinical practice. Prospective studies are needed to confirm these results. Based on this paucity of data, it is prudent to rely on the results demonstrated in A-HeFT and the subgroup analyses from V-HeFT I and V-HeFT II.
Final Recommendations:
- Despite the clear mortality benefit of HYD/ISDN demonstrated in black patients with NYHA Class III-IV HFrEF, utilization of this therapy in clinical practice remains inadequate. There must be greater efforts to improve prescribing of HYD/ISDN in this patient population as well as in those with HFrEF who can not take an ACEi, angiotensin II receptor blocker or angiotensin receptor-neprilysin inhibitor.
- Pharmacokinetic data do not indicate any clear differences between nitrate exposure when comparing BiDil®, ISDN, or ISMN. However, no clinical outcome data exists to suggest that extended-release ISMN is an appropriate alternative. The substitution of ISMN for ISDN is not recommended.
- In the absence of prospective comparator studies, it is likely appropriate to substitute the individual components of HYD and ISDN for brand name BiDil® especially if cost is of concern.
 |
Michael Plazak, PharmD
|
 |
Kristin Watson, PharmD, BCPS-AQ Cardiology
|
References:
- Cohn JN, Archibald DG, Ziesche S, et al. Effect of Vasodilator Therapy on Mortality in Chronic Congestive Heart Failure. N Eng J Med. 1986;314(24):1547-1552. doi:10.1056/NEJM198606123142404
- Cohn JN, Johnson G, Ziesche S, et al. A comparison of enalapril with hydralazine-isosorbide dinitrate in the treatment of chronic congestive heart failure. N Engl J Med. 1991;325(5):303-310. doi:10.1056/NEJM199108013250502
- Committee Members, Hunt SA, Baker DW, et al. ACC/AHA Guidelines for the Evaluation and Management of Chronic Heart Failure in the Adult: Executive Summary A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Revise the 1995 Guidelines for the Evaluation and Management of Heart Failure). Circulation. 2001;104(24):2996-3007. doi:10.1161/hc4901.102568
- Taylor AL, Ziesche S, Yancy C, et al. Combination of isosorbide dinitrate and hydralazine in blacks with heart failure. N Engl J Med. 2004;351(20):2049-2057. doi:10.1056/NEJMoa042934
- Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA Guideline for the Management of Heart Failure: Executive Summary. J Am Coll Cardiol. 2013;62(16):1495-1539. doi:10.1016/j.jacc.2013.05.020
- Yancy CW, Jessup M, Bozkurt B, et al. 2017 ACC/AHA/HFSA Focused Update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. Circulation. 2017;136(6). doi:10.1161/CIR.0000000000000509
- Golwala HB, Thadani U, Liang L, et al. Use of hydralazine-isosorbide dinitrate combination in African American and other race/ethnic group patients with heart failure and reduced left ventricular ejection fraction. J Am Heart Assoc. 2013;2(4):e000214. doi:10.1161/JAHA.113.000214
- Yancy CW, Fonarow GC, Albert NM, et al. Adherence to guideline-recommended adjunctive heart failure therapies among outpatient cardiology practices (findings from IMPROVE HF). Am J Cardiol. 2010;105(2):255-260. doi:10.1016/j.amjcard.2009.08.681
- Wolters Kluwer Clinical Drug Information, Inc. (Lexi-Drugs). Wolters Kluwer Clinical Drug Information, Inc.; 2019.
- Walmart. $4 Prescriptions. https://www.walmart.com/cp/4-dollar-prescriptions/1078664 Accessed May 15, 2019.
- Arbor® Pharmaceuticals, LLC. The Shaq BiDil (isosorbide dinitrate/hydralazine HCl) Access Program. https://www.shaqgetsreal.com/ Accessed May 15, 2019.
- Yancy CW, Januzzi JL, Allen LA, et al. 2017 ACC Expert Consensus Decision Pathway for Optimization of Heart Failure Treatment: Answers to 10 Pivotal Issues About Heart Failure With Reduced Ejection Fraction: A Report of the American College of Cardiology Task Force on Expert Consensus Decision Pathways. J Am Coll Cardiol. 2018;71(2):201-230. doi:10.1016/j.jacc.2017.11.025
- Ofili E, Anand I, Williams RA, Akinboboye O, Xu L, Puckrein G. Fixed-Dose Versus Off-Label Combination of Isosorbide Dinitrate Plus Hydralazine Hydrochloride: Retrospective Propensity-Matched Analysis in Black Medicare Patients with Heart Failure. Adv Ther. 2017;34(8):1976-1988. doi:10.1007/s12325-017-0584-x
- Hare JM. Nitroso-redox balance in the cardiovascular system. N Engl J Med. 2004;351(20):2112-2114. doi:10.1056/NEJMe048269
- Carson P, Ziesche S, Johnson G, Cohn JN. Racial differences in response to therapy for heart failure: analysis of the vasodilator-heart failure trials. Vasodilator-Heart Failure Trial Study Group. J Card Fail. 1999;5(3):178-187.
- Tam SW, Sabolinski ML, Worcel M, Packer M, Cohn JN. Lack of bioequivalence between different formulations of isosorbide dinitrate and hydralazine and the fixed-dose combination of isosorbide dinitrate/hydralazine: the V-HeFT paradox. Clin Pharmacokinet. 2007;46(10):885-895. doi:10.2165/00003088-200746100-00006
- Isosorbide mononitrate, extended-release [package insert]. Eatontown, NJ: Heritage Pharmaceuticals Inc.; 2017.
- Fung HL. Do nitrates differ? Br J Clin Pharmacol. 1992;34 Suppl 1:5S-9S. doi:10.1111/j.1365-2125.1992.tb04141.x
Share this post:

If the results from a study like that of the Scandinavian Simvastatin Survival Study (4S) can be applied to patients of all racial and/or ethnic backgrounds, then why are the results from the A-HeFT study only applicable to “self-identified blacks”? By the same logic of the 4S in which the patients were exclusively Scandinavian, why should we not apply the findings from the A-HeFT to patients of all racial and/or ethnic backgrounds (note: all patients in A-HeFT study were self-identified blacks)? The bigger question I ask is what relevance does race have in biology?…rhetorical question given that there is absolutely no biological basis for race. BiDil was not originally designed as a pharmacogenetic variant drug for black patients; it merely failed to show benefit to ACEi in the first round of the approval process. Subsequently, in the interest of a NIH initiate in the early/mid-90s to improve under-representation in biomedical research, NitroMed did it’s part to try and show there was benefit of BiDil in a “subgroup” analysis (i.e. self-identified blacks). BiDil was ultimately pushed through as the first race-based FDA approved pharmaceutical and made its way into practice without us (myself included) asking if it was right…without asking if the approval of this medication was truly going to reduce health disparities in the black population with HF.