Share this post:
Author: Sandeep Devabhakthuni, PharmD, BCCP
Patients with active cancer are at 7-fold increased risk of venous thromboembolism (VTE) and have a 2- to 6-fold increased likelihood of VTE-related death.1,2 Up to one-third of VTE diagnoses have been documented as cancer-associated, a known marker of poor prognosis in this population.3,4 Low molecular weight heparins (LMWH) are considered first-line for cancer-associated VTE given previous studies demonstrating superior efficacy over warfarin in reducing risk of recurrent VTE.5,6 However, one limitation with LMWH therapy is the need for parenteral administration, which is not always ideal in the outpatient setting. In the past decade, oral anticoagulation has evolved with the widespread use of direct oral anticoagulants (DOACs) in both VTE and atrial fibrillation and are often recommended over warfarin in specific populations.7-9 This blog summarizes the available data and provides recommendations for VTE treatment in patients with cancer.
Patients with active cancer are at increased risk of venous thromboembolic events, particularly in those with certain characteristics.10 Patients with aggressive solid tumors at advanced stages are at higher risk for VTE. Additionally, cancer type (e.g., pancreatic, brain, and lung) and specific gene mutations (e.g., JAK2 mutations) are strongly correlated with thrombotic risk.11,12 Moreover, numerous chemotherapy agents are also associated with increased risk of VTE, including platinum-based (e.g., cisplatin), hormonal (e.g., tamoxifen), anti-VEGF (e.g., bevacizumab), and immunomodulator (e.g., thalidomide) therapies.13
DOAC Evidence in VTE Treatment
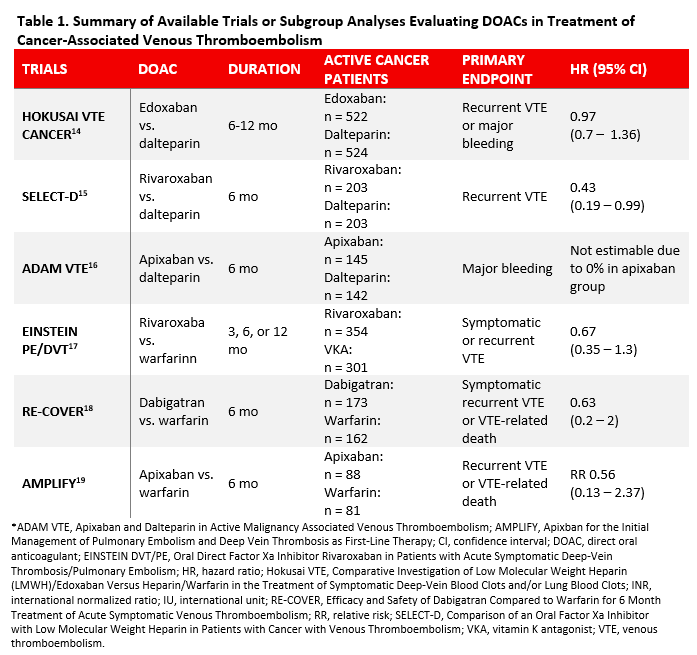
Low molecular weight heparins have previously been the standard of care in cancer-associated VTE. Evidence supporting LMWH over vitamin K antagonists (VKAs) is based on two large randomized, controlled trials.5,6 However, patients may not prefer LMWH over oral anticoagulation due to parenteral administration.14,15 To-date, there have been three head-to-head randomized, controlled trials that have compared DOAC with LMWH in cancer patients newly diagnosed with VTE and 3 subgroup analyses of cancer patients published from the landmark phase III trials evaluating DOACs. 16-21 These trials have been summarized in Table 1.
Edoxaban
Edoxaban was the first DOAC to be studied in cancer patients newly diagnosed with VTE. The Hokusai VTE Cancer trial was an open-label, randomized, non-inferiority trial that included 1050 cancer patients with VTE who received LMWH for at least 5 days followed by either edoxaban or dalteparin.16 Edoxaban was non-inferior to dalteparin in the primary composite endpoint (HR 0.97; 95% CI, 0.7-1.36; p = 0.006 for non-inferiority, p = 0.87 for superiority). Edoxaban had a nonsignificantly lower rate of recurrent VTE (HR 0.71; 95% CI, 0.48 to 1.06) but significantly higher rate of bleeding (HR 1.77; 95% CI, 1.03 to 3.04) driven by higher bleeding rates in patients with gastrointestinal cancers (13.2% vs. 2.4%, p = 0.02). Significant limitations included low incidence of primary outcome events and higher median duration with edoxaban compared to dalteparin (211 days vs. 184 days, p = 0.01).
Rivaroxaban
The SELECT-D Pilot trial was a multicenter, randomized, open-label trial of 406 patients with active cancer and symptomatic VTE who received either dalteparin or rivaroxaban for 6 months.17 Rivaroxaban reduced the primary outcomes of VTE recurrence compared to dalteparin (HR 0.43; 95% CI, 0.19 to 0.99). The 6-month cumulative rate of major bleeding was similar between groups (HR 1.83; 95% CI, 0.68 to 4.96). However, rivaroxaban was associated with higher rates of clinically relevant nonmajor bleeding (HR 3.76; 95% CI, 1.63-8.69). Limitations of this trial included short follow-up period of 6 months, lower event rate, and small sample size given that it was a pilot study.
There was also a published pooled subgroup analysis of 655 patients with active cancer from the landmark phase III EINSTEIN-PE/DVT trials.19 The rate of recurrent VTE was similar between groups (HR 0.67; 95% CI, 0.35 to 1.3), and rivaroxaban had lower risk of major bleeding (HR 0.42; 95% CI, 0.18 to 0.99). However, it is important to note that only 7.9% of the patients enrolled in the two phase III trials had active cancer, which limits these findings. There are also ongoing trials comparing rivaroxaban with LMWH including CONKO-11, CASTA-DIVA, and PRIORITY trials.22-24
Apixaban
The ADAM VTE trial, which was a multicenter, randomized, open-label, superiority trial, included 300 patients with cancer-associated VTE who received either apixaban or dalteparin for 6 months.18 The primary outcome of major bleeding was similar between the two groups (0% in apixaban group vs. 1.4% in dalteparin group, p = 0.138). Recurrent VTE was significantly lower with apixaban compared to dalteparin (HR 0.099; 95% CI, 0.013 to 0.78). Also, more patients randomized to dalteparin switched therapy compared to apixaban (22 vs. 6; p = 0.0012). Significant limitations include small sample size, high discontinuation rate in both groups due to patient dissatisfaction, and low event rates.
There was also a subgroup analysis of the phase III AMPLIFY trial that compared apixaban with enoxaparin followed by warfarin for VTE in 169 patients with active cancer.21 Apixaban had a similar rate of recurrent VTE (relative risk [RR] 0.56; 95% CI, 0.13 to 2.37) and major bleeding (RR 0.45; 95% CI, 0.08 to 2.46). Of note, only 3.1% of the patients in this trial had active cancer. There is another ongoing randomized trial called the CARAVAGGIO trial comparing apixaban to dalteparin.25
Dabigatran
A pooled subgroup analysis from two phase III trials (RE-COVER I and II) compared dabigatran to warfarin for acute VTE in 355 patients with cancer, representing 7% of patients included.20 There was no significant difference in efficacy between dabigatran and warfarin for cancer at baseline (HR 0.75; 95% CI, 0.2 to 2.8) or diagnosed during the study (HR 0.63; 95% CI, 0.2 to 2.8). Major bleeding was also similar in both groups during the study (HR 0.53; 95% CI, 0.29 to 1.1). The ongoing CANVAS trial will be comparing four DOACs (dabigatran, rivaroxaban, apixaban, or edoxaban) to LMWH, which will provide further insight into the composite efficacy and safety profile and drug-specific versus class effects.26
Guideline Recommendations for DOAC Use in Treatment of VTE
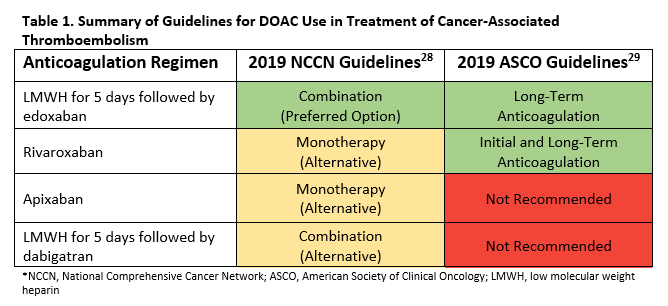
Until recently, guidelines recommended LMWH as the only first-line option for treatment of cancer-associated VTE. With the emerging data on DOAC use, the latest International Society on Thrombosis and Haemostasis guidance support use of DOACs in this population with preference for edoxaban and rivaroxaban if acute VTE and low risk of bleeding.27 For those at high risk of bleeding (e.g., gastrointestinal and genitourinary cancers), LMWH remains preferred for acute VTE.
Figure 1 summarizes the recent addition of recommendations for DOAC use in cancer-associated thromboembolism in the 2019 National Comprehensive Cancer Network (NCCN) Cancer-Associated Venous Thromboembolic Disease guidelines and 2019 American Society of Clinical Oncology (ASCO) VTE Prophylaxis and Treatment guidelines.28,29
Recommendations:
Despite the emerging data for DOACs, LMWH remains a first-line option in cancer-associated VTE given the robust evidence supporting its use.5,6 However, recent data from the Hokusai VTE, SELECT-D Pilot, and ADAM VTE trials suggest that edoxaban, rivaroxaban, and apixaban have similar efficacy compared to dalteparin, respectively.16-18 These data also suggest that patients with gastrointestinal cancer are at higher risk of bleeding when a DOAC is used. Therefore, it is reasonable to limit use of DOACs in cancer patients to those with low risk of bleeding. Data for therapeutic anticoagulation in the treatment of cancer-associated VTE beyond 6 months are limited to LMWH only.30 No recommendations for dabigatran can be made at this time due to limited data.
Special Considerations:
In cancer patients with progressive renal disease (CrCl < 15 mL/min), switching from a DOAC to an alternative anticoagulant with less renal cleance is preferred over anti-Xa level monitoring until further research is conducted.31 Finally, in those who develop thrombocytopenia, which is commonly associated with chemotherapy, anticoagulation should be avoided when platelet counts fall below 50,000 to 70,000/µL.32
Bottom Line:
Patients with active cancer are at a higher risk of VTE depending on cancer type and cancer therapies used.1-3 Until recently, LMWH therapy was the mainstay for VTE treatment in patients with active cancer given its superiority over VKAs.5,6 However, real-world data suggest that patients have suboptimal medication adherence with LMWH therapy.14,15 Recently published data suggests that edoxaban, rivaroxaban, and apixaban may have similar efficacy to LMWH.16-18 Therefore, DOACs represent a convenient alternative for VTE treatment in patients with active cancer at low risk of bleeding.27 Ultimately, a discussion between the patient and the multidisciplinary healthcare team is necessary; anticoagulation strategies should be highly individualized based on patient preferences and thrombotic and bleeding risk factors.
 |
Sandeep Devabhakthuni, PharmD, BCCP
|
References:
- Noble S, Pasi J. Epidemiology and pathophysiology of cancer-associated thrombosis. Br J Cancer. 2010;102:S2-9.
- Streiff MB, Holmstrom B, Angelini D, et al. NCCN guidelines insights: cancer-associated venous thromboembolic disease, version 2.2018. J Natl Compr Canc Netw. 2018;16:1289-1303.
- Puurunen MK, Gona PN, Larson MG, Murabito JM, Magnani JW, O’Donnell CJ. Epidemiology of venous thromboembolism in the Framingham Heart Study. Thromb Res. 2016;145:27-33.
- Faller N, Limacher A, Mean M, et al. Predictors and causes of long-term mortality in elderly patients with acute venous thromboembolism: a prospective cohort study. Am J Med. 2017;130:198-206.
- Lee AYY, Levine MN, Baker RI, et al. Low-molecular-weight heparin versus a coumarin for the prevention of recurrent venous thromboembolism in patients with cancer. N Engl J Med. 2003;349:146-53.
- Lee AY, Kamphuisen PW, Meyer G, et al. Tinzaparin vs warfarin for treatment of acute venous thromboembolism in patients with active cancer: a randomized clinical trial. JAMA. 2015;314:677-86.
- Mosarla RC, Vaduganathan M, Qamar A, Moslehi J, Piazza G, Giugliano RP. Anticoagulation strategies in patients with cancer. J Am Coll Cardiol. 2019;73:1336-49.
- Kearon C, Akl EA, Ornelas J, et al. Antithrombotic therapy for VTE disease: CHEST guidelines and expert panel report. Chest. 2016;149:315-52.
- Writing Group Members, January CT, Wann LS, Calkins H, et al. 2019 AHA/ACC/HRS focused update of the 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. Heart Rhythm. 2019;16:e66-e93.
- Navi BB, Reiner AS, Kamel H, et al. Risk of arterial thromboembolism in patients with cancer. J Am Coll Cardiol. 2017;70:926-38.
- Khorana AA, Kuderer NM, Culakova E, Lyman GH, Francis CW. Development and validation of a predictive model for chemotherapy-associated thrombosis. Blood. 2008;111:4902-7.
- Edelmann B, Gupta N, Schnoder TM, et al. JAK2-V617F promotes venous thrombosis through beta1/beta2 integrin activation. J Clin Invest. 2018;128:4359-71.
- Moslehi JJ. Cardiovascular toxic effects of targeted cancer therapies. N Engl J Med. 2016;375:1457-67.
- Khorana AA, McCrae KR, Milentijevic D, Fortier J, et al. Current practice patterns and patient persistence with anticoagulant treatments for cancer-associated thrombosis. Res Pract Thromb Hemost. 2017;1:14-22.
- Streiff MB, Milentijevic D, McCrae K, et al. Effectiveness and safety of anticoagulants for the treatment of venous thromboembolism in patients with cancer. Am J Hematol. 2018;93:664-71.
- Raskob GE, van Es N, Verhamme P, et al. Edoxaban for the treatment of cancer-associated venous thromboembolism. N Engl J Med. 2018;378:615-24.
- Young AM, Marshall A, Thirlwall J, et al. Comparison of an oral factor Xa inhibitor with low molecular weight heparin in patients with cancer with venous thromboembolism: results of a randomized trial (SELECT-D). J Clin Oncol. 2018;36:2017-23.
- McBane R 2nd, Wysokinski WE, Le-Rademacher JG, et al. Apixaban and dalteparin in active malignancy associated venous thromboembolism: the ADAM VTE trial. J Thromb Haemost. 2019. DOI: 10.1111/jth.14662. [Epub ahead of print]
- Prins MH, Lensing AW, Brighton TA, et al. Oral rivaroxaban versus enoxaparin with vitamin K antagonist for the treatment of symptomatic venous thromboembolism in patients with cancer (EINSTEIN-DVT and EINSTEIN-PE): a pooled subgroup analysis of two randomised controlled trials. Lancet Haemotol. 2014;1:e37-46.
- Schulman S, Goldhabet SZ, Kearon C, et al. Treatment with dabigatran or warfarin in patients with venous thromboembolism and cancer. Thromb Haemost. 2015;114:150-157.
- Agnelli G, Buller HR, Cohen A, et al. Oral apixaban for the treatment of venous thromboembolism in cancer patients: results from the AMPLIFY trial. J Thromb Haemost. 2015;13:2187-91.
- gov [Internet]. Bethesda (MD): National Libarary of Medicine (US). 2015 Oct 22 – . Identifier NCT02583191. Rivaroxaban in the treatment of venous thromboembolism (VTE) in cancer patients. 2019 Apr 18 [cited 2019 Nov 18]. Available from: https://clinicaltrials.gov/ct2/show/NCT02583191.
- gov [Internet]. Bethesda (MD): National Libarary of Medicine (US). 2016 Apr 21 – . Identifier NCT02746185. Cancer associated thrombosis, a pilot treatment study using rivaroxaban (CASTA-DIVA). 2018 Aug 13 [cited 2019 Nov 18]. Available from: https://clinicaltrials.gov/ct2/show/NCT02746185.
- gov [Internet]. Bethesda (MD): National Libarary of Medicine (US). 2017 May 4 – . Identifier NCT03139487. A randomized phase II study to compare the safety and efficacy of dalteparin vs. rivaroxaban for cancer associated venous thromboembolism (PRIORITY). 2017 May 4 [cited 2019 Nov 18]. Available from: https://clinicaltrials.gov/ct2/show/NCT03139487.
- Agnelli G, Becattini C, Bauersachs R, et al. Apixaban versus dalteparin for the treatment of acute venous thromboembolism in patients with cancer: the CARAVAGGIO study. Thromb Haemost. 2018;118:1668-78.
- gov [Internet]. Bethesda (MD): National Libarary of Medicine (US). 2016 Apr 20 – . Identifier NCT02744092. Direct oral anticoagulants (DOACs) versus LMWH +/- warfarin for VTE in cancer (CANVAS). 2019 Nov 13 [cited 2019 Nov 18]. Available from: https://clinicaltrials.gov/ct2/show/NCT02744092.
- Khorana AA, Noble S, Lee AYY, et al. Role of direct oral anticoagulants in the treatment of cancer-associated venous thromboembolism: guidance from the SSC of the ISTH. J Thromb Haemost. 2018;16:1891-4.
- Streiff MB, Holmstrom B, Angelini D, et al. NCCN Clinical Practice Guidelines in Oncology: cancer-associated venous thromboembolic disease. National Comprehensive Cancer Network. 2019 Feb 28. https://www.nccn.org/professionals/physician_gls/pdf/vte.pdf.
- Key NS, Khorona AA, Kuderer NM, et al. Venous thromboembolism prophylaxis and treatment in patients with cancer: ASCO clinical practice guideline update. J Clin Oncol. 2019;5:JCO1901461 DOI: 10.1200/JCO.19.01461. [Epub ahead of print].
- Francis CW, Kessler CM, Goldhaber SZ, et al. Treatment of venous thromboembolism in cancer patients with dalteparin for up to 12 months: the DALTECAN Study. J Thromb Haemost. 2015;13:1028-35.
- Witt DM, Nieuwlaat R, Clark NP, et al. American Society of Hematology 2018 guidelines for management of venous thromboembolism: optimal management of anticoagulation therapy. Blood Adv. 2018;2:3257-91.
- Kearon C, Akl EA, Onelas J, et al. Antithrombotic therapy for VTE Disease: CHEST guideline and expert panel report. Chest. 2016;149:315-52.
Share this post:

Thanks for the wonderful post!
According to NCCN guidelines, full dose enoxaparin can be given as long as the platelet count is >50,000.
With a count of 25,000 to 50,000 the dose should be reduced to half, and with a count of less than 25,000 the drug should be temporarily held.
Thank you for the clarification! This was not mentioned in the blog since the focus was on DOAC use. Agree with the recommendation for LMWH.