Share this post:
Author: Stormi Gale, PharmD, BCCP
Recently, the results of VICTORIA (Vericiguat Global Study in Subjects with Heart Failure with Reduced Ejection Fraction) were reported at the American College of Cardiology Virtual World Congress.1 In short, this study assessed the efficacy and safety of vericiguat, a soluble guanylate cyclase (sGC) stimulator in patients with heart failure with reduced ejection fraction (HFrEF) (Figure 1). Although vericiguat met its primary composite outcome of death from cardiovascular causes or first hospitalization for heart failure, this was driven primarily by a reduction in heart failure (HF) hospitalizations (27.4% vs. 29.6%; HR, 0.90; 95% CI, 0.81 to 1.00). Despite a technically positive study, I’m not sure vericiguat is worth the hype for several reasons: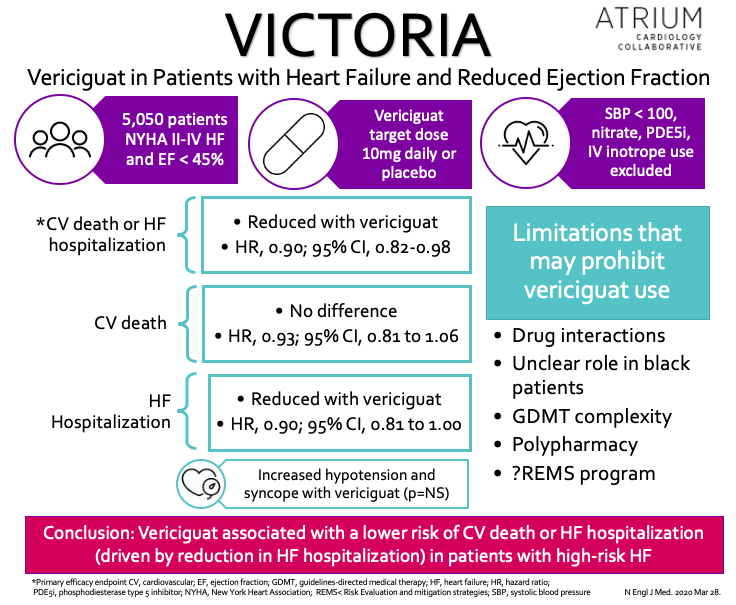
Drug-drug interactions
There was an important reason why patients on PDE5 inhibitors and long-acting nitrates were excluded from VICTORIA: sGC stimulators are contraindicated with these medications due to an increased risk of hypotension. Although it isn’t uncommon to consider drug-drug interactions when initiating new therapies, having to avoid these agents is especially concerning in this population, as described in further detail below.
PDE5 inhibitors: In my experience, patients may not always be forthcoming with their cardiologist about being on a PDE5 inhibitor, which may result in inadvertent but inappropriate prescribing. Additionally, patients may intermittently prioritize their PDE5 inhibitor over HFrEF therapies (not understanding that this interaction doesn’t exist with all guideline-directed medical therapy [GDMT]), leading to medication nonadherence. Encouraging open communication and emphasizing education may mitigate some of these issues, but the potential for adverse events certainly exists.
Nitrates: It is particularly unfortunate that one of the contraindicated medications with sGC inhibitors is one of the most commonly used therapies in patients with HFrEF.2,3 Long-acting oral nitrates have known survival benefit in black patients, which relates to another issue with VICTORIA to be described in further below. Furthermore, many patients (of all races) with acute decompensated heart failure (ADHF) are initiated on intravenous nitrates for preload/afterload reduction and oral nitrates are often temporarily substituted for renin-angiotensin-aldosterone inhibitors in patients with transient worsening of renal function during episodes of ADHF.4 Patients on vericiguat prior to admission would be at an increased risk of adverse events early in their hospitalization, especially if sGC therapy is held and therefore unable to trigger alerts for drug-drug interactions when new orders for nitrate therapy are entered.
Unclear role in black patients
As we have detailed in previous blogs, black patients are notoriously underrepresented in HFrEF studies and VICTORIA was no exception. Not only did this population comprise less than 5% of patients enrolled in the study, there are serious implications for not being able to use nitrates concomitantly with vericiguat, as the combination of hydralazine/isosorbide dinitrate (ISDN) decreases mortality in black patients with HFrEF (check out a previous blog for more information on this).2 Conversely, I would argue that this population may stand to benefit the most from vericiguat, given its effects on nitric oxide and known benefits of targeting this pathway. Would the reductions in mortality have been different if the population was comprised of 50% black patients? Could vericiguat have provided us a once daily option for mortality reduction in this population?
Nonetheless, because of VICTORIA’s low representation of black patients (and a primary endpoint driven by hospitalizations without an independent reduction in mortality), it is imperative that we do not prioritize vericiguat over hydralazine/ISDN in black patients unless there is some other reason that this combination is not being used (e.g., frequency of dosing).
Polypharmacy and overcomplication of HFrEF therapy
Our therapeutic options for patients with HFrEF have expanded significantly over the past 10 years, and now include several more costly medications that have been shown to reduce mortality [e.g., angiotensin receptor-neprilysin inhibitor (ARNI), sodium glucose co-transporter 2 inhibitors (SGLT2i)].3,4 As a result, there is already uncertainty regarding when to add additional therapies (especially those that decrease both hospitalizations and mortality). Furthermore, vericiguat was associated with an increased risk of hypotension and syncope, a well-described limitation to up-titration of GDMT, particularly therapies shown to improve survival. Adding vericiguat to a patient’s medication regimen may prohibit the optimization of these therapies, which are already known to be suboptimally dosed in this population.5 Along with this and the growing concern for how polypharmacy effects these patients (e.g., expenses, adherence), when would the benefits of adding vericiguat outweigh the opportunity costs?
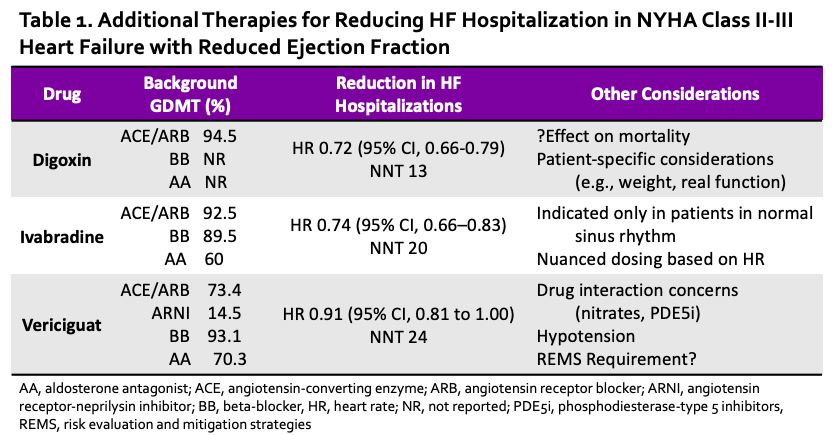
Other drugs reduce hospitalizations, too
Notwithstanding the many agents that have been shown to reduce mortality in HFrEF, digoxin and ivabradine have both been shown to independently reduce hospitalizations (Table 1).6,7 In a previous blog, we discussed the merits of using digoxin over ivabradine for this purpose, including a potential mortality benefit when kept within the therapeutic range). Regardless, my feelings toward vericiguat are similar to those of ivabradine. What does vericiguat offer over digoxin (and even ivabradine), other than additional complexity, risk of drug-drug interactions, and an increased risk of hypotension? Furthermore, riociguat, the only currently FDA-approved sCG stimulator, requires Risk Evaluation and Mitigation Strategies (REMS) enrollment due to its teratogenicity. Assuming the same for vericiguat, this adds another layer of complexity, as prescribers, pharmacies, and female patients must be enrolled in this program to prescribe/dispense/use sGC stimulators (and here we thought prior authorizations were time-consuming – imagine if monthly pregnancy tests were required for other types of GDMT!). Some might argue that recent trials represent contemporary HFrEF therapy, which is certainly true when comparing VICTORIA to the DIG trial. However, the percentages of concomitant GDMT used in SHIFT and VICTORIA were similar, and despite ARNI being available during the VICTORIA trial, its use was still quite low (<15%).
 Bottom Line
Bottom Line
The pharmacotherapy of HFrEF has increased in complexity over the past several years, and vericiguat seems poised to continue this trend. However, its real-world utility is limited by the risk of adverse events and drug-drug interactions and its potential to preclude the optimization of life-saving GDMT. It’s prudent that when evaluating these new therapies we ask ourselves: help or hype? In the case of vericiguat, I think hype.
 |
Stormi Gale, PharmD, BCCP
|
Reviewed by: Brent N. Reed, PharmD, BCCP
References
- Armstrong PW, Pieske B, Anstrom KJ, et al. Vericiguat in Patients with Heart Failure and Reduced Ejection Fraction. N Engl J Med. 2020;0(0):null.
- Yancy CW, Fonarow GC, Albert NM, et al. Adherence to Guideline-Recommended Adjunctive Heart Failure Therapies Among Outpatient Cardiology Practices (Findings from IMPROVE HF). Am J Cardiol. 2010;105(2):255-260.
- Thomas KL, Hernandez AF, Dai D, et al. Association of race/ethnicity with clinical risk factors, quality of care, and acute outcomes in patients hospitalized with heart failure. Am Heart J. 2011;161(4):746-754.
- Hollenberg SM, Stevenson LW, Ahmad T, et al. 2019 ACC Expert Consensus Decision Pathway on Risk Assessment, Management, and Clinical Trajectory of Patients Hospitalized With Heart Failure: A Report of the American College of Cardiology Solution Set Oversight Committee. J Am Coll Cardiol. 2019;74(15):1966-2011.
- Combination of Isosorbide Dinitrate and Hydralazine in Blacks with Heart Failure | NEJM.
- Angiotensin–Neprilysin Inhibition versus Enalapril in Heart Failure | NEJM.
- McMurray JJV, Solomon SD, Inzucchi SE, et al. Dapagliflozin in Patients with Heart Failure and Reduced Ejection Fraction. N Engl J Med. 2019;381(21):1995-2008.
- Greene SJ, Butler J, Albert NM, et al. Medical Therapy for Heart Failure With Reduced Ejection Fraction: The CHAMP-HF Registry. J Am Coll Cardiol. 2018;72(4):351-366.
- The Effect of Digoxin on Mortality and Morbidity in Patients with Heart Failure. N Engl J Med. 1997;336(8):525-533.
- Ivabradine and outcomes in chronic heart failure (SHIFT): a randomised placebo-controlled study – The Lancet.
Share this post:
