Share this post:
Author: Michael E. Plazak, PharmD, BCCP
Survival following continuous-flow left ventricular assist device (LVAD) implantation continues to improve and currently exceeds 85% at one year.1 However, device-related complications still impair quality of life and limit duration of support. Device thrombosis is a potentially devastating complication that occurs in approximately 4-13% of patients with axial-flow devices, such as the HeartMate II, and 8-9% of patients with centrifugal-flow devices, such as the HeartWare LVAD.2–4 The incidence of pump thrombosis in the HeartMate 3, a newer-generation centrifugal flow device, appears to be much lower (1.4% at two years).5 Nevertheless, consequences can be severe and can range from end-organ dysfunction to hemodynamic compromise and death.
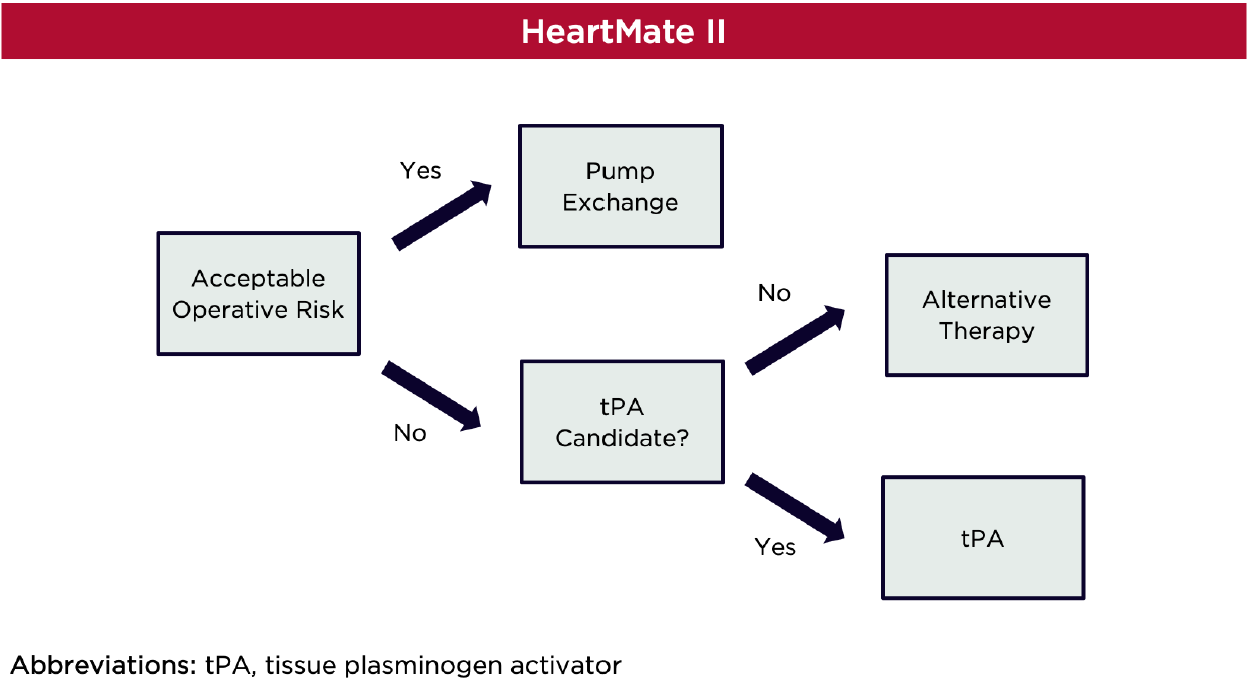
Strategies for the management of pump thrombosis have traditionally focused on escalation of antithrombotic therapy, but efficacy has varied and bleeding complications are frequent.6–10 Studies have indicated that surgical pump exchange results in higher treatment success, lower mortality, and lower recurrence rate compared to medical therapy, but these outcomes should be interpreted cautiously given significant heterogeneity in devices, protocols, and sample sizes.11–13 International Society of Heart and Lung Transplantation guidelines suggest thrombolysis as a potential alternative to device exchange in those with end-organ dysfunction and unacceptable operative risk.14
Thrombus Generation
Thrombus formation within the LVAD is influenced by several key factors including: activation of the extrinsic coagulation pathway via the blood-titanium alloy interaction; hypercoagulability mediated by activated platelets; and aberrant blood flow patterns that result in sheer-stress, hemolysis, and stasis.15,16 Differences in device engineering result in distinct patterns of thrombus formation and propagation. Thrombosis of the HeartMate II is frequently characterized by globular clot formations on the the inflow bearings and regions of sharp angulation on the inflow and outflow grafts.17,18 Pump thrombogenesis also appears to be more insidious in the HeartMate II as evidenced by delayed detection of clinically-significant hemolysis.19 HeartWare device thrombosis is typically characterized by laminar fibrin formation on the impeller.20 The exceptionally low incidence of pump thrombosis with the HeartMate 3 device is attributed to several features that reduce shear stress including wider blood-flow passages, friction-free movement via full magnetic levitation, and intrinsic pulsatility.
Thrombolysis Outcomes with the HeartMate II Device
Thrombolytic therapy in HeartMate II recipients has traditionally been considered a last-line treatment option given the delayed detection of clinically significant thrombosis and overall success of early pump exchange.17,19,21,22 Additionally, several single-center experiences have detailed mixed success with use of thrombolytics in this population. For example, use of 20-30 mg boluses of tissue plasminogen activator (tPA) resulted in low rates of thrombus resolution in a cohort of 37 patients, 64.8% of which had a HeartMate II device.23 Only 11.5% achieved successful resolution of device thrombosis; eighteen patients required subsequent pump exchange. Two experienced intracranial hemorrhage (ICH) following tPA. Lack of tPA efficacy may have been explained by high lactate dehydrogenase and plasma-free hemoglobin on presentation, indicating higher degrees of hemolysis and presence of larger thrombus burden.
In a second study consisting mostly of patients with HeartMate II devices (n = 20), continuous infusion tPA was associated with acceptable outcomes.24 This protocol consisted of a 5-mg intravenous bolus of tPA, followed by a 3 mg/hr infusion for 10 hours. Resolution of hemolysis with no requirement for device exchange occurred in 70% of those receiving tPA and survival was 95% at 30 days. Only 10% of patients experienced ICH.
Catheter-directed tPA has also been proposed as an approach to reduce systemic thrombolytic exposure. Unfortunately, this strategy has also resulted in poor response and safety. In a case series of eight HeartMate II recipients, intraventricular tPA only resolved hemolysis in three patients.25 Of the remaining patients, one required subsequent device exchange, two experienced embolic strokes, and one suffered ICH followed by recurrent device thrombosis. Three of these patients died following intraventricular tPA.
Thrombolysis Outcomes with the HeartWare Device
Early experience with tPA in the HeartWare population also demonstrated variable efficacy and safety. A retrospective analysis of the HeartWare bridge-to-transplant and subsequent continued-access protocol trials revealed that tPA successfully resolved device thrombosis in 63% of those treated (n = 31).3 Six of these patients ultimately required device exchange. Limitations included heterogeneity in the medical therapy and thrombolysis protocols used across centers (e.g., tPA alone or in combination with heparin or GPIIb/IIIa inhibitors) and lack of clarity regarding dosing and bleeding outcomes. A second study representing a single-center experience described successful implementation of continuous infusion tPA 5 mg/hr until pump power and flow resolved.26 Short-term resolution of device thrombosis was achieved in all nine patients after a median of 9 (2.75-19.25) hours of tPA infusion. Three patients had recurrence of pump thrombosis and one patient experienced a non-fatal ICH.
The HeartWare log file system allows for real-time identification of occlusion, ingestion, and build-up of thrombus based on pump power, flow, and speed, which are automatically recorded by the controller at fifteen minute intervals.27–30 Percent expected power ((maximum power/expected power) x 100) and growth rate ((maximum power – baseline power)/time to reach maximum power)) are useful calculations that can be used to identify thrombus kinetics (i.e., gradual build-up vs. sudden thrombus formation). When used in combination, these parameters can aid in identifying suitable candidates for tPA therapy. In one study, a growth rate < 1 and a percentage expected power < 200% were associated with a higher incidence of medical therapy success, whereas a growth rate > 1 and percent expected power > 200% were associated with treatment failure.31 In a similar analysis, HeartWare log files were used to investigate the efficacy of tPA in 52 pump thrombus events in 43 patients.32 Treatment was successful in 57% of the overall cohort, but 81.3% in those with percent expected power < 200% and growth rate < 1.25. If at least one of those thresholds was not met, tPA was only successful 14.3% of the time (p<0.001). Interestingly, there was no difference in time to tPA administration between those who failed tPA and those who did not (5.4 days vs. 4.7 days, p=0.92) These results suggest that gradual thrombus formation and minor power spikes are more amenable to thrombolysis in those with a HeartWare device.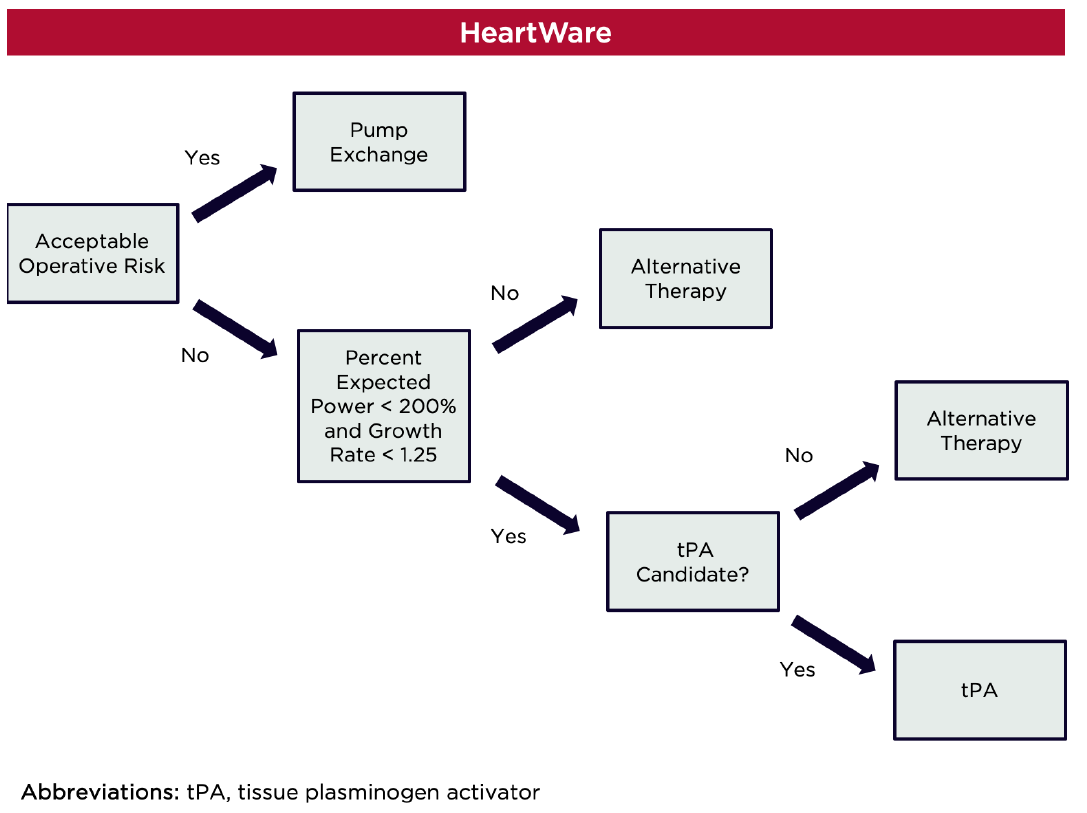
Bottom Line
Publication of high-quality studies in this area is unlikely and thus, small experiences must be used to guide treatment decisions. In patients with acceptable operative risk, pump exchange should be prioritized in patients with a HeartMate II device, especially since minimally-invasive approaches have reduced complications.11–13,33 Thrombolysis may be considered as an alternative in those who are not surgical candidates. In patients with thrombosis of a HeartWare device, tPA may be considered in those with log files that demonstrate percent expected power < 200% and growth rate < 1.25. If both of these parameters are not met, pump exchange should be prioritized.
The appropriate timing and dosing of thrombolytics following onset of pump thrombosis are perhaps the largest unknowns. Early thrombolytic administration was thought to be paramount as older and more organized thrombi may be more resistant to lysis. However, no difference in time to tPA administration was observed among those who failed tPA and those who did not in a HeartWare population.32 Thus, thrombus kinetics and organization may hold more precedence. Nonetheless, tPA administration should be expedited once the decision to proceed with thrombolysis is made. Dosing of tPA is exceptionally variable across LVAD centers. Continuous infusion of alteplase appears to achieve the best balance between safety and efficacy as bolus regimens have been associated with higher rates of treatment failure and ICH. There is insufficient evidence to support intraventricular thrombolytic administration at this time. The efficacy of thrombolytics when used in combination with other antithrombotic therapies (e.g., GP IIb/IIIa inhibitors) is largely unknown. A systematic review and meta-analysis demonstrated that thrombolytics, used alone or in combination with either heparin, direct thrombin inhibitors, or a GP IIb/IIIa inhibitor, completely resolved pump thrombosis in 66% of patients.10 However, there appeared to be no additional efficacy when thrombolytics were combined with more than one therapy and major bleeding occurred more frequently.
In summary, the use of thrombolytics for LVAD pump thrombosis is nuanced and many questions remain. Additionally, the incidence of pump thrombosis is likely to decline in the coming years with increasing implantation of HeartMate 3 devices and incremental improvements in hemocompatibility. In those patients who remain on older-generation devices, there appears to be a role for thrombolytics, but therapy should be selected carefully based on both patient- and pump-specific characteristics.
 |
Michael Plazak, PharmD, BCCP
|
Reviewed by: Brent N. Reed, PharmD, BCCP
References
- Teuteberg JJ, Cleveland JC, Cowger J, et al. The Society of Thoracic Surgeons Intermacs 2019 Annual Report: The Changing Landscape of Devices and Indications. Ann Thorac Surg. 2020;109(3):649-660. doi:10.1016/j.athoracsur.2019.12.005
- Starling RC, Moazami N, Silvestry SC, et al. Unexpected abrupt increase in left ventricular assist device thrombosis. N Engl J Med. 2014;370(1):33-40. doi:10.1056/NEJMoa1313385
- Najjar SS, Slaughter MS, Pagani FD, et al. An analysis of pump thrombus events in patients in the HeartWare ADVANCE bridge to transplant and continued access protocol trial. J Heart Lung Transplant. 2014;33(1):23-34. doi:10.1016/j.healun.2013.12.001
- Baumann Kreuziger LM, Kim B, Wieselthaler GM. Antithrombotic therapy for left ventricular assist devices in adults: a systematic review. J Thromb Haemost. 2015;13(6):946-955. doi:10.1111/jth.12948
- Mehra MR, Uriel N, Naka Y, et al. A Fully Magnetically Levitated Left Ventricular Assist Device — Final Report. New England Journal of Medicine. 2019;380(17):1618-1627. doi:10.1056/NEJMoa1900486
- Bitar A, Vijayakrishnan R, Lenneman A, et al. The Use of Eptifibatide Alone or in Combination With Heparin or Argatroban for Suspected Thrombosis in Patients With Left Ventricular Assist Devices. Artif Organs. 2017;41(12):1092-1098. doi:10.1111/aor.12910
- Oliveira GH, Al-Kindi SG, ElAmm C, et al. Platelet Inhibition with Ticagrelor for Left Ventricular Assist Device Thrombosis. Circ Heart Fail. 2015;8(3):649-651. doi:10.1161/CIRCHEARTFAILURE.115.002096
- Wert L, Hanke JS, Dogan G, et al. Argatroban administration as therapy for thrombosis in patients with continuous-flow ventricular assist devices. J Thorac Dis. 2018;10(Suppl 15):S1720-S1727. doi:10.21037/jtd.2017.10.164
- Sylvia LM, Ordway L, Pham DT, DeNofrio D, Kiernan M. Bivalirudin for treatment of LVAD thrombosis: a case series. ASAIO J. 2014;60(6):744-747. doi:10.1097/MAT.0000000000000122
- Dang G, Epperla N, Muppidi V, et al. Medical Management of Pump-Related Thrombosis in Patients with Continuous-Flow Left Ventricular Assist Devices: A Systematic Review and Meta-Analysis. ASAIO J. 2017;63(4):373-385. doi:10.1097/MAT.0000000000000497
- Luc JGY, Tchantchaleishvili V, Phan K, Dunlay SM, Maltais S, Stulak JM. Medical Therapy As Compared To Surgical Device Exchange for Left Ventricular Assist Device Thrombosis: A Systematic Review and Meta-Analysis. ASAIO J. 2019;65(4):307-317. doi:10.1097/MAT.0000000000000833
- Koda Y, Kitahara H, Kalantari S, et al. Surgical device exchange provides improved clinical outcomes compared to medical therapy in treating continuous-flow left ventricular assist device thrombosis. Artif Organs. 2020;44(4):367-374. doi:10.1111/aor.13594
- Oezpeker C, Zittermann A, Ensminger S, et al. Systemic Thrombolysis Versus Device Exchange for Pump Thrombosis Management: A Single-Center Experience. ASAIO Journal. 2016;62(3):246–251. doi:10.1097/MAT.0000000000000340
- Goldstein DJ, John R, Salerno C, et al. Algorithm for the diagnosis and management of suspected pump thrombus. J Heart Lung Transplant. 2013;32(7):667-670. doi:10.1016/j.healun.2013.05.002
- de Biasi AR, Manning KB, Salemi A. Science for surgeons: understanding pump thrombogenesis in continuous-flow left ventricular assist devices. J Thorac Cardiovasc Surg. 2015;149(3):667-673. doi:10.1016/j.jtcvs.2014.11.041
- Jennings DL, Weeks PA. Thrombosis in continuous-flow left ventricular assist devices: pathophysiology, prevention, and pharmacologic management. Pharmacotherapy. 2015;35(1):79-98. doi:10.1002/phar.1501
- Kirklin JK, Naftel DC, Kormos RL, et al. Interagency Registry for Mechanically Assisted Circulatory Support (INTERMACS) analysis of pump thrombosis in the HeartMate II left ventricular assist device. J Heart Lung Transplant. 2014;33(1):12-22. doi:10.1016/j.healun.2013.11.001
- Capoccia M, Bowles CT, Sabashnikov A, Simon A. Recurrent Early Thrombus Formation in HeartMate II Left Ventricular Assist Device. J Investig Med High Impact Case Rep. 2013;1(2). doi:10.1177/2324709613490676
- Cowger JA, Romano MA, Shah P, et al. Hemolysis: a harbinger of adverse outcome after left ventricular assist device implant. J Heart Lung Transplant. 2014;33(1):35-43. doi:10.1016/j.healun.2013.08.021
- Aissaoui N, Börgermann J, Gummert J, Morshuis M. HeartWare continuous-flow ventricular assist device thrombosis: the Bad Oeynhausen experience. J Thorac Cardiovasc Surg. 2012;143(4):e37-39. doi:10.1016/j.jtcvs.2011.12.035
- Uriel N, Han J, Morrison KA, et al. Device thrombosis in HeartMate II continuous-flow left ventricular assist devices: a multifactorial phenomenon. J Heart Lung Transplant. 2014;33(1):51-59. doi:10.1016/j.healun.2013.10.005
- Levin AP, Uriel N, Takayama H, et al. Device exchange in HeartMate II recipients: long-term outcomes and risk of thrombosis recurrence. ASAIO J. 2015;61(2):144-149. doi:10.1097/MAT.0000000000000170
- Seese L, Hickey G, Keebler M, Thoma F, Kilic A. Limited Efficacy of Thrombolytics for Pump Thrombosis in Durable Left Ventricular Assist Devices. The Annals of Thoracic Surgery. Published online April 26, 2020. doi:10.1016/j.athoracsur.2020.03.061
- Nair N, Schmitt AA, Rau EM, Anders S, Sandler D, Icenogle TB. Thrombolytics in VAD management – A single-center experience. Int J Cardiol Heart Vasc. 2016;11:49-54. doi:10.1016/j.ijcha.2016.03.006
- Schlendorf KH, Zalawadiya S, Shah AS, et al. Early outcomes using hepatitis C-positive donors for cardiac transplantation in the era of effective direct-acting anti-viral therapies. J Heart Lung Transplant. 2018;37(6):763-769. doi:10.1016/j.healun.2018.01.1293
- Schrage B, Grahn H, Wagner FM, et al. Effective treatment with a new protocol using tissue-type plasminogen activator thrombolysis for pump thrombosis with the HVAD device. Eur Heart J Acute Cardiovasc Care. 2018;7(8):766-770. doi:10.1177/2048872616688418
- Chorpenning K, Brown MC, Voskoboynikov N, Reyes C, Dierlam AE, Tamez D. HeartWare controller logs a diagnostic tool and clinical management aid for the HVAD pump. ASAIO J. 2014;60(1):115-118. doi:10.1097/MAT.0000000000000022
- Kamouh A, John R, Eckman P. Successful treatment of early thrombosis of HeartWare left ventricular assist device with intraventricular thrombolytics. Ann Thorac Surg. 2012;94(1):281-283. doi:10.1016/j.athoracsur.2011.12.024
- Santise G, Sciacca S, Baglini R, Clemenza F, Pilato M. Can learning to interpret pump messages help lead to an early diagnosis of HeartWare ventricular assist device thrombosis? ASAIO J. 2012;58(6):629-632. doi:10.1097/MAT.0b013e31826a87bc
- Kiernan MS, Pham DT, DeNofrio D, Kapur NK. Management of HeartWare left ventricular assist device thrombosis using intracavitary thrombolytics. J Thorac Cardiovasc Surg. 2011;142(3):712-714. doi:10.1016/j.jtcvs.2010.11.022
- Stulak JM, Dunlay SM, Sharma S, et al. Treatment of device thrombus in the HeartWare HVAD: Success and outcomes depend significantly on the initial treatment strategy. J Heart Lung Transplant. 2015;34(12):1535-1541. doi:10.1016/j.healun.2015.10.020
- Jorde UP, Aaronson KD, Najjar SS, et al. Identification and Management of Pump Thrombus in the HeartWare Left Ventricular Assist Device System: A Novel Approach Using Log File Analysis. JACC Heart Fail. 2015;3(11):849-856. doi:10.1016/j.jchf.2015.06.015
- Soleimani B, Price LC, Stephenson ER, El-Banayosy A, Pae WE. Outcomes of Minimally Invasive Approach for Exchange of the HeartMate II (HMII) Left Ventricular Assist Device (LVAD). The Journal of Heart and Lung Transplantation. 2014;33(4):S201. doi:10.1016/j.healun.2014.01.882
Share this post: