Share this post:
Author: Zachary R. Noel, PharmD, BCCP
Multiple studies have been published evaluating antithrombotic strategies in patients following transcatheter aortic valve replacement (TAVR) since the release of the 2017 American Heart Association (AHA)/American College of Cardiology (ACC) Focused Update on for Management of Valvular Heart Disease. This blog serves as an update to our prior blog and offers recommendations for post-TAVR antithrombotic therapy in patients with concomitant atrial fibrillation and recent coronary artery stenting.
Recent Trials Evaluating Post-TAVR Antithrombotic Therapy
The GALILEO trial randomized patients who did not have a pre-existing indication for anticoagulation to either standard dual antiplatelet therapy (i.e., aspirin 75-100 daily and clopidogrel 75 mg daily) or dual therapy with rivaroxaban 10 mg once daily and aspirin 75-100 mg daily following TAVR.2 After 3 months, aspirin 81-100 mg monotherapy was continued in the antiplatelet arm and rivaroxaban 10 mg was continued in the anticoagulation arm. The study was discontinued prematurely due to an increase in all-cause death (5.8 versus 3.4 deaths per 100 person-years) in those receiving rivaroxaban 10 mg. The reason for the increase in death, which was driven by non-cardiovascular causes, remains unclear. There was no difference in the primary bleeding endpoint (defined as VARC life-threatening, disabling, or major bleeding), though there was an increase in combined TIMI major and minor bleeding as well as ISTH major bleeding. Also of note, a sub study evaluating rates of subclinical leaflet motion abnormalities, which may be a surrogate maker for subclinical leaflet thrombosis and risk of cerebrovascular events, showed fewer leaflet motion abnormalities in those in the anticoagulation arm.3 Taken altogether, it remains unclear why an increase in death was observed.
The more recently published POPular TAVI trial involved two cohorts of patients.3-4 Cohort A consisted of patients without an indication for therapeutic anticoagulation; patients were randomly assigned to aspirin 80-100 mg or standard dual antiplatelet therapy (aspirin plus clopidogrel). Unsurprisingly, monotherapy with aspirin resulted in significantly less overall bleeding than dual antiplatelet therapy (15.1% vs 26.6%; HR 0.57, 95 % CI 0.42-0.77) as well as less non-procedure-related bleeding (15.1% vs 24.9%; HR 0.61, 95% CI 0.44-0.83). Importantly, there was no signal for increased thromboembolic events (though the trial was not powered to detect this).
POPular TAVI cohort B included patients post-TAVR with an indication for anticoagulation (atrial fibrillation for 95% of those enrolled). Patients were randomized to anticoagulation monotherapy, with a direct oral anticoagulation (DOAC) or vitamin K antagonist (VKA, goal INR 2), or to combination therapy with clopidogrel and a DOAC or VKA. After 3 months, anticoagulation monotherapy was continued in all patients. Patients were followed for 12 months. The most noteworthy exclusion criterion was recent coronary stent placement (defined as <3 months from drug eluting stent placement or 1 month for bare metal stents). Over 40% had pre-existing coronary artery disease; however, fewer than 10% had a history of myocardial infarction. Approximately 75% received a VKA and 25% received a DOAC (data on the specific DOAC and dosing was not reported). The primary composite endpoint of all bleeding was significantly lower in the anticoagulation monotherapy group (21.7% vs 34.6%; HR 0.63, 95% CI 0.43-0.90), as was non-procedural related bleeding (21.7% vs 34.0%; HR 0.64, 95% CI 0.44-0.92). Rates of thromboembolic complications, including stroke and MI, were not significantly different between the groups. Subgroup analyses suggest similar or improved outcomes in those receiving a DOAC, but this is merely exploratory.
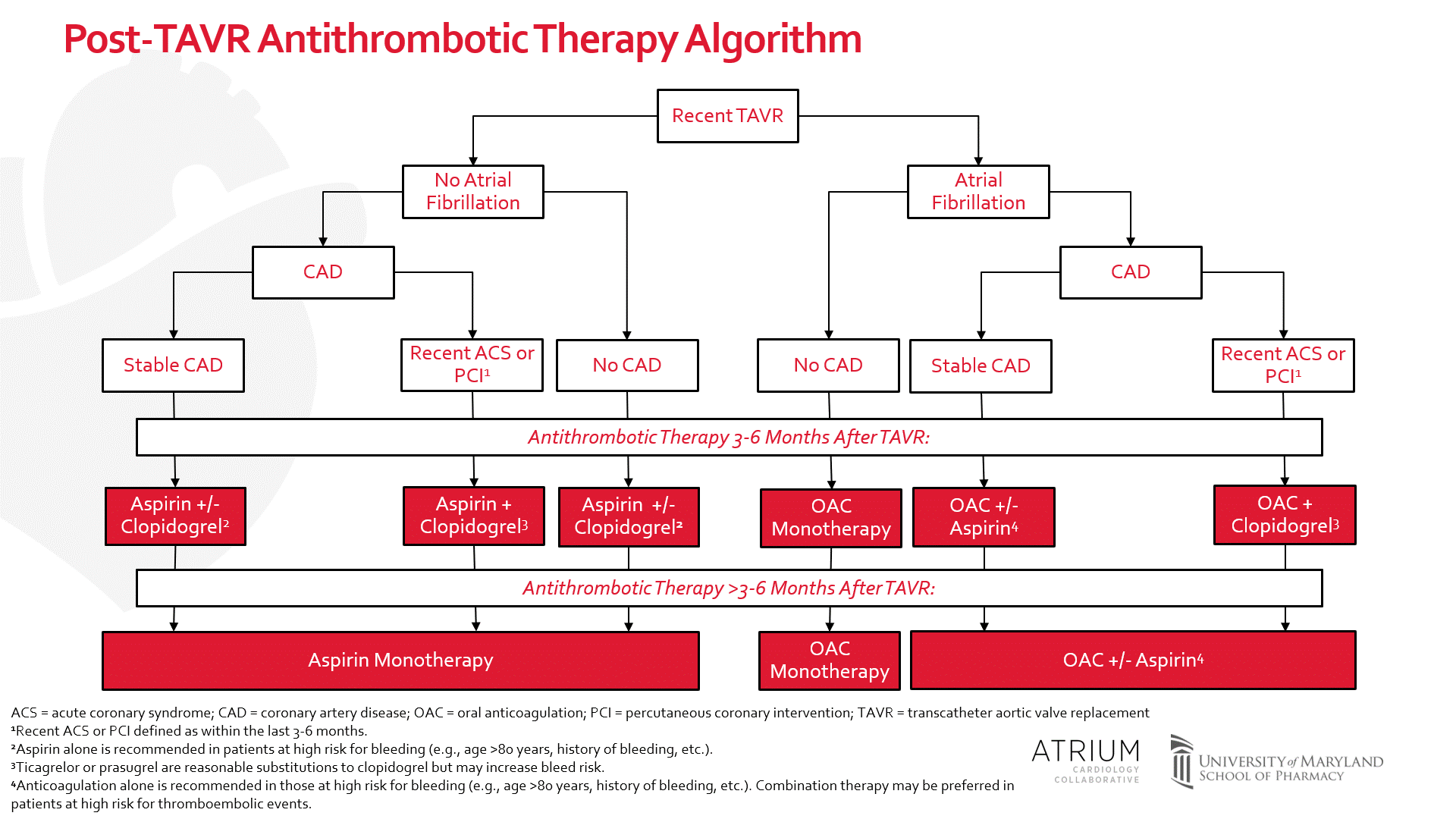
Recommendations for Post-TAVR Antithrombotic Therapy
For patients without a compelling indication for anticoagulation, what antithrombotic regimen should be selected after TAVR?
Taken altogether, the results of GALILEO do not support the role of anticoagulation in patients following TAVR who do not otherwise have an indication for anticoagulation. Data from POPular-TAVI cohort A provides supportive evidence for single antiplatelet therapy following TAVR; however, this trial was not powered to detect a difference in thromboembolic events. For this reason, single or dual antiplatelet therapy is reasonable (Figure 1). Choosing a less, or more, aggressive antiplatelet regimen should be decided based on bleeding and thromboembolic risk factors. Patients with recent coronary stent placement or at high-risk of cardiovascular events should be considered for dual antiplatelet therapy. On the contrary, patients at high risk of bleeding (e.g., age > 80 years old, history of prior bleeding events, etc.) may be better suited for less aggressive antiplatelet therapy.
For patients with a compelling indication for anticoagulation (e.g., atrial fibrillation), what antithrombotic regimen should be selected after TAVR?
POPular TAVI cohort B provides supporting evidence for anticoagulation alone following TAVR in select populations with atrial fibrillation (Figure 1). Patients with atrial fibrillation without coronary artery disease, or atrial fibrillation with stable coronary artery disease, are reasonable candidates for anticoagulation monotherapy. On the contrary, patients with atrial fibrillation at high-risk for cardiovascular events or who have a recent coronary stent should also receive aspirin or clopidogrel. Importantly, triple therapy (i.e., aspirin, a P2Y12 inhibitor, and an anticoagulant) should be avoided.
Closing Thoughts
Recent data from GALILEO and POPular TAVI have provided insight on antithrombotic regimens after TAVR. We provide an updated algorithm for selecting an antithrombotic regimen after TAVR in Figure 1.
 |
Zachary R. Noel, PharmD, BCCP
|
Reviewed by: Kristin Watson, PharmD, BCCP
References
- Nishimura RA, Otto CM, Bonow RO, et al. Clinical Practice Guideline: Focused Update: 2017 AHA/ACC Focused Update of the 2014 AHA/ACC Guideline for the Management of Patients With Valvular Heart Disease. A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Journal of the American College of Cardiology. 2017;70:252-289. doi:10.1016/j.jacc.2017.03.011.
- Dangas GD, Tijssen JGP, Wöhrle J, et al. A controlled trial of rivaroxaban after transcatheter aortic-valve replacement. N Engl J Med 2020;382:120-9.
- De Backer O, Dangas G, Jilaihawi H, Leipsic J, GALILEO-4D Investigators;, et al; Reduced Leaflet Motion after Transcatheter Aortic-Valve Replacement. New England Journal of Medicine (USA). 382(Feb):130. Accessed October 15, 2020.
- Nijenhuis VJ, Brouwer J, Delewi R, et al. Anticoagulation with or without Clopidogrel after Transcatheter Aortic-Valve Implantation. New England Journal of Medicine. 2020;382(18):1696-1707. doi:10.1056/NEJMoa1915152.
- Brouwer J, Nijenhuis VJ, Delewi R, et al. Aspirin with or without Clopidogrel after Transcatheter Aortic-Valve Implantation. The New England Journal of Medicine. August 2020. doi:10.1056/NEJMoa2017815
Share this post: