Share this post:
Author: Stormi Gale, PharmD, BCPS, BCCP
The long-awaited 2018 American College of Cardiology/American Heart Association (ACC/AHA) cholesterol guidelines were released at AHA 2018 in Chicago.1 This update can be described as an amalgamation of three previous sets of guidelines: 1) 2013 ACC/AHA guidelines, 2) 2017 ACC/AHA non-statin therapy update, and 3) the National Lipid Association (NLA) recommendations.2–5 As you might expect from an effort to combine such varying recommendations, the final product is a lot to process. Fewer recommendations are black-and-white, although arguably for good reason. Below we highlight some of the most important changes in the latest update:
The four statin benefit groups are out (sort of).
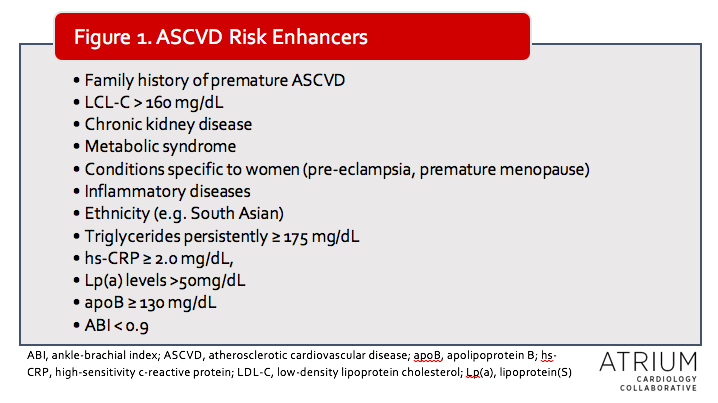
Like many other therapeutic areas, cholesterol management is moving away from a one-size-fits-all strategy. Whereas prior guidelines made recommendations largely based on the four statin benefit groups and 10-year atherosclerotic cardiovascular disease (ASCVD) risk, the 2018 update takes risk-stratification a step further. The new guidelines give increased consideration to individual risk factors (referred to as ASCVD risk enhancers) and less emphasis on ASCVD risk calculations alone. The limitations of risk-stratification systems have been well-described, as population risk factors do not necessarily reflect individual risk. The authors of the update note that certain baseline risk factors, such as genetics (not included in the risk calculator), play an important role in individualized risk. Thus, these are now included as additional considerations for management, particularly for those at intermediate risk.
For example, according to the 2013 guidelines, primary prevention patients with ASCVD risk of at least 7.5% (without DM) would have been prescribed a moderate to high intensity statin.2 Now, patients with ASCVD risk ≥ 7.5% but <20% will get a moderate intensity statin only if “ASCVD Risk Enhancers” are present (see table). Additionally, coronary artery calcium scoring can be used in patients in whom the risk/benefit remains uncertain. Risk discussions are now emphasized in all patients without ASCVD, DM, or elevated baseline low-density lipoprotein cholesterol (LDL-C), regardless of ASCVD risk.
Patients ages 40 to 75 years with DM no longer require ASCVD risk calculation at all; moderate-intensity statins are recommended for every patient with DM (with the option for high-intensity if warranted by an individualized risk assessment).
Higher-intensity statins remain recommended for patients with a history of ASCVD and those with (LDL-C) ≥ 190, in addition to primary prevention patients with ASCVD risk 20% or greater.
Non-statin therapies are in (for some).
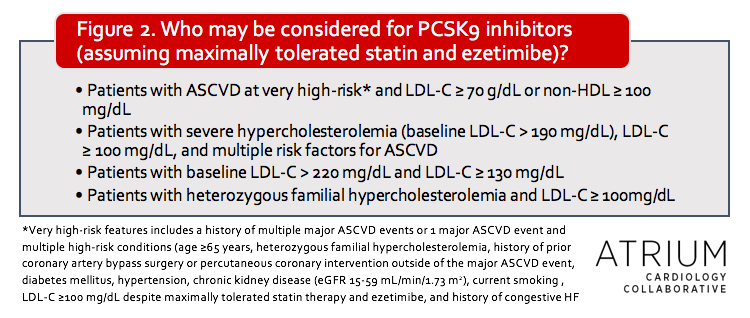
The 2013 ACC/AHA guidelines did not comment on non-statin therapies, although an expert consensus decision pathway was released several years later.3 In the 2018 update, considerations are given for which patients should be considered for non-statin therapies (see Figure 2), but with a greater emphasis on clinical judgment of individual risk compared to previous recommendations.
It is unsurprising that the most recent updates include recommendations for these agents given the growing data supporting their use. Shortly after the publication of the 2013 guidelines, IMPROVE-IT revealed an improvement in cardiovascular (CV) outcomes with the addition of ezetimibe in patients with recent ACS (and a mean baseline LDL-C of 94 mg/dL), although a notable limitation of this study was the background use of moderate- rather than high-intensity statin therapy.6 The FOURIER trial found that the addition of proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitor therapy in patients with stable ASCVD and LCL-C ≥ 70 g/dL despite optimized statins reduced the primary endpoint of composite CV death, myocardial infarction, stroke, hospitalization for unstable angina, or coronary revascularization.7 The benefits were seen even in the subgroup of patients with lowest baseline LDL-C (median 74 mg/dL). More recently, the long-awaited ODYSSEY OUTCOMES trial was released and included patients on high intensity or maximally tolerated stain therapy with recent ACS (within 1 to 12 months), LDL-C ≥ 70 mg/dL, non-HDL ≥ 100 mg/dL, or apoB ≥ 80mg/dL.8 Despite a mean baseline LDL-C of 92 mg/dL, patients randomized to alirocumab experienced a decrease in the composite primary outcome of death from coronary heart disease, nonfatal myocardial infarction, fatal or nonfatal ischemic stroke, or unstable angina requiring hospitalization.
Another item that was addressed in this update is the cost considerations for PCSK9 inhibitor therapy, which was done through the use of value statements. The authors note that most pharmacoeconomic evaluations have suggested that these agents have low value as a result of their high expense. For this reason, these agents should only be utilized in those with the highest CV risk (See Figure 2 for specific populations mentioned in guidelines). As mentioned previously, even high-risk patients should generally receive ezetimibe in addition to maximum statin therapy prior to consideration of PCSK9 inhibitors, and the decision to use these therapies should include patient-clinician discussion about risks, benefits and costs.
LDL-C goals are back.
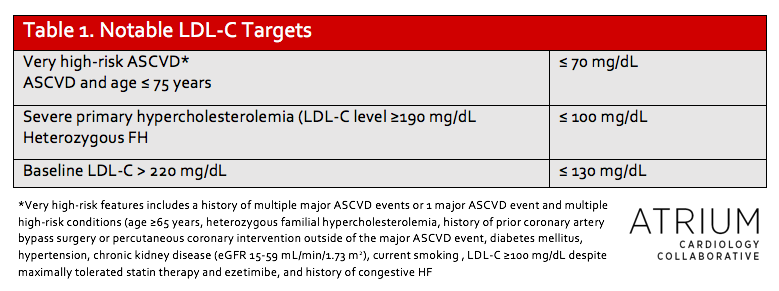
One of the greatest areas of controversy in the 2013 guidelines was the removal of LDL-C goals, although these remained a therapeutic target in the NLA guidelines and percent LDL-C-lowering was a major focus of the 2017 focused update.3–5 The primary argument for retaining some reference to LDL-C-lowering is the residual CV risk (ranging from 10-30%) in patients despite treatment with a high-intensity statin as well as an improvement in outcomes when LDL-C is lowered beyond previous thresholds.9–11 Consequently, the 2018 guidelines recommend specific percent LDL-C-lowering for most patients and also specify LDL-C targets of < 70mg/dL in very high-risk patients with a history of ASCVD, and < 100 mg/dL in patients with baseline LDL-C ≥190 mg/dL. Both of these targets can be achieved with the addition of ezetimibe or PCSK9 inhibitors as discussed above.
So in regards to LDL-C, how low is too low? Historically, many have been apprehensive regarding the risk of adverse events with intensive LDL-C lowering; however, recent trials have assuaged this concern. In IMPROVE-IT, patients who achieved LDL-C < 30 mg/dL had no increase in adverse events compared to those with higher LDL-C concentrations. Similarly, no harm was observed in the subgroup of patients in FOURIER who achieved LDL-C < 10 mg/dL. Thus, in high-risk populations, the benefits of additional non-statin therapies appear to outweigh the risks. More studies are needed to determine the risks and benefits associated with extreme LDL-C lowering, particularly given the small sample of patient who achieved such levels in the above studies and the reported role of cholesterol in several basic physiologic functions.12
Bottom Line
Overall, this update not only reflects new evidence from the past several years but also the changing landscape of cholesterol therapy. As mentioned previously, contemporary management of blood cholesterol and overall CV risk reduction with both statin and non-statin therapies continues to increase in complexity. Previous guidelines simplified cholesterol treatment by assigning specific intensities to varying benefit groups and did not require adjustments in therapy (in the absence of new risk factors or adverse effects). The 2018 update not only tasks providers with a greater responsibility for evaluating patients’ overall risk, but also for following up to assess if targets have been achieved. Due to the increasing complexity of cholesterol management and the need to individualize therapy, it is reasonable to expect an increase in referrals to lipid specialists.
 |
Stormi Gale, PharmD, BCPS
|
References:
- Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Journal of the American College of Cardiology. November 2018:25708.
- Stone NJ, Robinson JG, Lichtenstein AH, et al. 2013 ACC/AHA Guideline on the Treatment of Blood Cholesterol to Reduce Atherosclerotic Cardiovascular Risk in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129(25 suppl 2):S1-S45.
- Lloyd-Jones DM, Morris PB, Ballantyne CM, et al. 2017 Focused Update of the 2016 ACC Expert Consensus Decision Pathway on the Role of Non-Statin Therapies for LDL-Cholesterol Lowering in the Management of Atherosclerotic Cardiovascular Disease Risk: A Report of the American College of Cardiology Task Force on Expert Consensus Decision Pathways. Journal of the American College of Cardiology. 2017;70(14):1785-1822.
- Jacobson TA, Ito MK, Maki KC, et al. National Lipid Association Recommendations for Patient-Centered Management of Dyslipidemia: Part 1—Full Report. Journal of Clinical Lipidology. 2015;9(2):129-169.
- Jacobson TA, Maki KC, Orringer CE, et al. National Lipid Association Recommendations for Patient-Centered Management of Dyslipidemia: Part 2. Journal of Clinical Lipidology. 2015;9(6):S1-S122.e1.
- Cannon CP, Blazing MA, Giugliano RP, et al. Ezetimibe Added to Statin Therapy after Acute Coronary Syndromes. New England Journal of Medicine. 2015;372(25):2387-2397.
- Sabatine MS, Giugliano RP, Keech AC, et al. Evolocumab and Clinical Outcomes in Patients with Cardiovascular Disease. New England Journal of Medicine. 2017;376(18):1713-1722.
- Schwartz GG, Steg PG, Szarek M, et al. Alirocumab and Cardiovascular Outcomes after Acute Coronary Syndrome. New England Journal of Medicine. 2018;379(22):2097-2107.
- Serban M-C, Colantonio LD, Manthripragada AD, et al. Statin Intolerance and Risk of Coronary Heart Events and All-Cause Mortality Following Myocardial Infarction. J Am Coll Cardiol. 2017;69(11):1386-1395.
- Cannon CP, Braunwald E, McCabe CH, et al. Intensive versus Moderate Lipid Lowering with Statins after Acute Coronary Syndromes. http://dx.doi.org/101056/NEJMoa040583. October 2009.
- LaRosa JC, Grundy SM, Waters DD, et al. Intensive Lipid Lowering with Atorvastatin in Patients with Stable Coronary Disease. New England Journal of Medicine. 2005;352(14):1425-1435.
- Olsson AG, Angelin B, Assmann G, et al. Can LDL cholesterol be too low? Possible risks of extremely low levels. Journal of Internal Medicine. 2017;281(6):534-553.
Share this post:

Pingback: LITFL Review 355 • LITFL Medical Blog • FOAMed Review