Share this post:
Author: Zachary R. Noel, PharmD, BCCP
For years, I have advocated for preferential use of ticagrelor over other P2Y12 inhibitors in patients with acute coronary syndrome (ACS). It has a faster, more predictable antiplatelet effect compared to clopidogrel and (unlike prasugrel) demonstrated mortality benefit in the landmark PLATO trial.1 I’ve also cited many critical flaws in the landmark trials comparing prasugrel and clopidogrel.2,3 However, the recent results of The Intracoronary Stenting and Antithrombotic Regimen: Rapid Early Action for Coronary Treatment (ISAR-REACT) 5 trial have, to say the least, brought into question the notion that ticagrelor is a superior P2Y12 inhibitor in patients with ACS. This blog will briefly review the results of ISAR-REACT 5, but more importantly outline key considerations for the use of prasugrel in clinical practice.
Trial Overview
ISAR-REACT 5 was a randomized, multicenter, open-label trial comparing ticagrelor to prasugrel in patients presenting with ACS.4 For patients presenting with STEMI, ticagrelor 180 mg and prasugrel 60 mg were administered as soon as possible. For NSTEMI, ticagrelor 180 mg was administered upstream of coronary angiography. On the contrary, prasugrel 60 mg was administered provisionally only after coronary angiography. Patients received a maintenance dose of ticagrelor 90 mg twice daily or prasugrel 10 mg once daily (unless the patient’s age was ≥ 75 years or weight ≤ 60 kg, in which case prasugrel 5 mg daily was administered). The composite primary endpoint included death, myocardial infarction, or stroke at 1 year. Importantly, patients with a history of stroke or end stage renal disease were excluded, as were patients receiving therapeutic anticoagulation.
| Table 1: Strengths and Limitations of ISAR-REACT 5 | |
| Strengths | Limitations |
| Multi-center, randomized | Open-label |
| Timing of loading dose | 10-fold greater number of patients in prasugrel arm excluded from analysis (for unclear reasons) |
| Contemporary invasive strategies for ACS | |
| Intent-to-treat analysis | |
Over 4000 patients were randomized to ticagrelor or prasugrel. Approximately 80% of patients underwent PCI and approximately 40% had STEMI at presentation. At one year, there were more events in the ticagrelor arm compared to the prasugrel arm (9.3% vs 6.9%; 95% CI 1.09-1.70; p-value 0.006). The primary endpoint was driven by reductions in non-fatal myocardial infarction (4.8% vs 3.0%; 95% CI 1.18-2.25). There were no differences in all-cause death or death from cardiovascular causes, nor was there a difference in major bleeding complications, including hemorrhagic strokes.
Clinical Application of Prasugrel: Pros and Cons
While criticisms and limitations of ISAR-REACT 5 certainly exist (Table 1), the evidence for using prasugrel is compelling. As with any emerging data that requires a paradigm shift in our clinical practice, we must judiciously apply the evidence and consider additional factors such as cost, pill burden, and patient preference. Additionally, collateral effects on protocols and pathways must also be taken into account. Below are some key considerations for practitioners and institutions when evaluating the appropriateness of prasugrel.
The Up Side of Using Prasugrel
Cost
Unlike ticagrelor, prasugrel is a once daily medication and available generically for as little as $20-30 per month.5 This may be particularly advantageous in patients at high risk for recurrent thrombotic events and have barriers to accessing ticagrelor (ie, insurance coverage, copay, etc.).
Antiplatelet Switching
Similar to clopidogrel, prasugrel is a thienopyridine. This makes for easier antiplatelet switching and avoids the potential interaction between thienopyridines and ticagrelor.6 In the acute setting (ie, ACS), when switching from prasugrel to clopidogrel, the 2017 European Society of Cardiology Focused Update on Dual Antiplatelet Therapy in Coronary Artery Disease recommends giving a 600 mg loading dose of clopidogrel 24 hours after the previous prasugrel dose; however, this is a IIb recommendation and it may be reasonable to forgo the loading dose of clopidogrel since no pharmacodynamic interaction exists like it does with ticagrelor.6 Indeed, in the chronic setting, the guidelines suggest forgoing the loading dose and simply starting clopidogrel 75 mg once daily. When switching from clopidogrel to prasugrel in the acute setting, a loading dose of prasugrel can be given irrespective of timing of the last clopidogrel dose.
Adverse Effect Profile
Prasugrel is not associated with dyspnea or ventricular pauses and thus may have a more favorable adverse effect profile in select patients. Although there is more major bleeding with prasugrel than clopidogrel, based on the results of ISAR-REACT 5 it appears there is little difference in bleeding between prasugrel and ticagrelor.2,3
The Down Side of Using Prasugrel
Timing of Loading Dose
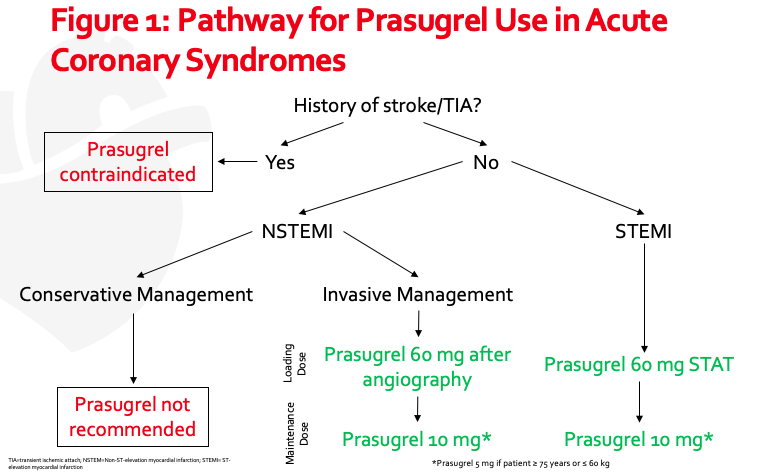
Previous data has shown that upstream administration of prasugrel in patients with NSTEMI results in more CABG- and non-CABG major bleeding (for more information on upstream P2Y12 inhibitor use click here).7 For this reason, patients with NSTEMI in ISAR-REACT 5 were administered prasugrel 60 mg after coronary angiography was performed. Institutions looking to incorporate prasugrel into their ACS pathways must take this into consideration. Patients who present with STEMI should still receive upstream prasugrel as soon as possible.
Dose Reduction
The maintenance dose of prasugrel in ISAR-REACT 5 was 10 mg daily, unless patients were ≥ 75 years or weighed ≤ 60 kg in which case a maintenance dose of 5 mg daily was used. It’s worth noting that this dosing is based on a number of pharmacokinetic and pharmacodynamic studies, but very little outcomes data exists.8 Although the exact number of patients receiving prasugrel 5 mg was not disclosed in ISAR-REACT 5, ~15-20% met criteria for this dose. Subgroup analyses of patients ≥ 75 years or ≤ 60 kg did not show benefit of prasugrel over ticagrelor, but given the small subset of patients the data is grossly underpowered. Taken altogether, prasugrel 10 mg should be avoided in older adults (ie, ≥ 75 years) or patients weighing ≤ 60 kg. Clopidogrel, ticagrelor, or prasugrel 5 mg remain reasonable options in these patients.
Medical Management
Prasugrel is not recommended in patients being medically managed for ACS. This data comes from the TRILOGY trial, in which prasugrel did not reduce MACE compared to clopidogrel in patients treated conservatively.2 Interestingly, in ISAR-REACT 5, patients were permitted to continue with a randomly assigned P2Y12 inhibitor (including prasugrel) following angiography even if PCI was not performed (though only ~5% of patients met this criterion). Clopidogrel or ticagrelor remain the preferred P2Y12 inhibitors in patients with ACS being conservatively managed.
Contraindications and Exclusions
Stroke/TIA: A history of stroke or transient ischemic attack is a contraindication for prasugrel use, and thus these patients were excluded from ISAR-REACT 5. This is a Class III recommendation for harm in the current NSTE-ACS guidelines.9
End Stage Renal Disease (ESRD): Patients with ESRD were excluded from ISAR-REACT 5. Because patients with ESRD have underlying platelet dysfunction and a predisposition towards bleeding, more potent P2Y12 inhibitors such as prasugrel or ticagrelor should be used with caution.
Anticoagulation: Importantly, patients with an indication for anticoagulation were excluded from this trial. Recent trials evaluating anticoagulation plus a P2Y12 inhibitor used almost exclusively clopidogrel, with ~10% using ticagrelor and virtually none using prasugrel (for more information on triple therapy click here). Prasugrel should therefore be used cautiously in patients receiving concurrent anticoagulation.
Conclusion
In light of the results of ISAR-REACT 5, a reflexive increase in prasugrel use is inevitable. While the results will (and should) be scrutinized, it is imperative that we reassess — or at least acknowledge — our biases and focus on judicious application to our patients. Deciding what antiplatelet medication to select, as well as duration, is complex and multifaceted. There is no “one size fits all” approach, highlighting the importance of a patient-centered, interdisciplinary approach to management.
 |
Zachary R. Noel, PharmD, BCCP
|
References
- Wallentin L, Becker RC, Budaj A, et al. Ticagrelor versus Clopidogrel in Patients with Acute Coronary Syndromes. N Engl J Med. 2009;361(11):1045-1057. doi:10.1056/NEJMoa0904327
- Roe MT, Armstrong PW, Fox KAA, et al. Prasugrel versus Clopidogrel for Acute Coronary Syndromes without Revascularization. N Engl J Med. 2012;367(14):1297-1309. doi:10.1056/NEJMoa1205512
- Wiviott SD, Braunwald E, McCabe CH, et al. Prasugrel versus Clopidogrel in Patients with Acute Coronary Syndromes. N Engl J Med. 2007;357(20):2001-2015. doi:10.1056/NEJMoa0706482
- Schüpke S, Neumann F-J, Menichelli M, et al. Ticagrelor or Prasugrel in Patients with Acute Coronary Syndromes. N Engl J Med. September 2019:NEJMoa1908973. doi:10.1056/NEJMoa1908973
- Prasugrel. https://www.goodrx.com/.
- Valgimigli M, Bueno H, Byrne RA, et al. 2017 ESC focused update on dual antiplatelet therapy in coronary artery disease developed in collaboration with EACTS. Eur Heart J. 2018;39(3):213-260. doi:10.1093/eurheartj/ehx419
- Montalescot G, Bolognese L, Dudek D, et al. Pretreatment with Prasugrel in Non–ST-Segment Elevation Acute Coronary Syndromes. N Engl J Med. 2013;369(11):999-1010. doi:10.1056/NEJMoa1308075
- Jakubowski JA, Erlinge D, Alexopoulos D, et al. The Rationale for and Clinical Pharmacology of Prasugrel 5 mg. Am J Cardiovasc Drugs. 2017;17(2):109-121. doi:10.1007/s40256-016-0202-3
- Amsterdam EA, Wenger NK, Brindis RG, et al. 2014 AHA/ACC Guideline for the Management of Patients With Non–ST-Elevation Acute Coronary Syndromes: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;130(25). doi:10.1161/CIR.0000000000000134
Share this post: